Difference between revisions of "Optical Microscopy Part 2: Fluorescence Microscopy"
MAXINE JONAS (Talk | contribs) |
m (Reverted edits by Steven Wasserman (talk) to last revision by Juliesutton) |
||
| (170 intermediate revisions by 6 users not shown) | |||
| Line 1: | Line 1: | ||
| − | |||
[[Category:20.309]] | [[Category:20.309]] | ||
[[Category:Optical Microscopy Lab]] | [[Category:Optical Microscopy Lab]] | ||
{{Template:20.309}} | {{Template:20.309}} | ||
| − | == | + | <br /> |
| − | = | + | <center> |
| + | {| | ||
| + | | style="color: black; background-color: black; width: 320px;" | | ||
| + | [[Image:3T3 Actin.png|300px]] | ||
| + | | style="color: black; background-color: black; width: 320px;" | | ||
| + | [[Image:3T3 Actin plus CytoD.png|300px]] | ||
| + | |- | ||
| + | !style="color: black; width: 320px;" | | ||
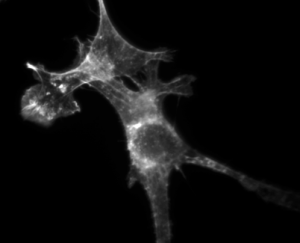
| + | 3T3 cells. Actin stained with Alexa Fluor 568 Phalloidin. | ||
| + | !style="color: black; width: 320px;" | | ||
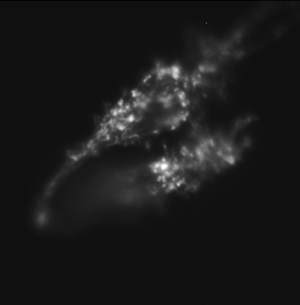
| + | Same cell type incubated with cytochalasin D, an inhibitor of actin polymerization, prior to fixing and stained. | ||
| + | |} | ||
| + | </center> | ||
| + | <br /> | ||
| − | + | ==Overview== | |
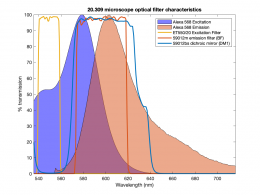
| + | [[Image:Alexa 568 and filters.png|thumb|260px|right|Excitation and emission spectra of Alexa 563. Green stripe indicates excitation laser wavelength of 532 nm. Shaded area shows passband of emission filter.]] | ||
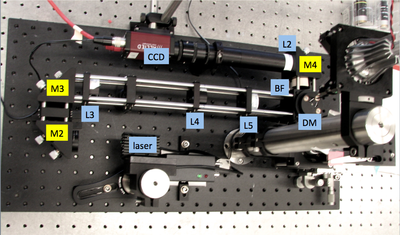
| + | In this part of the lab, you will add epifluorescence imaging capability to your microscope, make images of fluorescent samples, and process the images to correct artifacts caused by nonuniform illumination. The samples you will image include fluorescent microspheres of several sizes and cells stained with a fluorescent dye. A typical spectrum is shown on the right. The excitation light source is a 5 mW diode laser with a nominal wavelength of 532 nm — a striking, brilliant green. Emission is in the red/orange range. To make the correction for nonuniform illumination, you will also make images of a uniform fluorescence reference slide and a dark image with the illuminator turned off. | ||
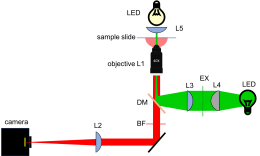
| − | + | [[Image:20.309 130911 YourMicroscope.png|thumb|260px|right|Fluorescence microscope block diagram.]] | |
| − | + | As shown in the block diagram, the major components required for fluorescence imaging are an illuminator (laser, L3, L4, and L5), dichroic mirror (DM), and emission filter (BF). The illuminator provides light in the appropriate wavelength range to excite the fluorescent molecules in the sample. Fluorescence microscopes that use broadand light sources such as arc lamps require an additional filter called an excitation filter to limit the wavelengths in the illumination to the proper range. Because lasers emit light in a very narrow range of wavelengths, an excitation filter is unnecessary. | |
| − | + | ||
| − | + | [[Image:20.309Hw3Imagespectra.JPG|right|thumb|260px|Transmission spectra for the 565DCXT dichroic and the E590LPv2 barrier filter]] | |
| + | Excitation light comes from beneath the sample, through the objective lens. A dichroic mirror directs the excitation toward the objective and sample. The mirror must reflect wavelengths in the excitation range and pass the longer wavelengths of the emitted fluorescence. In fluorescence imaging, illumination intensity is typically 5 or 6 orders of magnitude greater than emitted fluorescence, so it is crucial to filter out excitation photons as completely as possible. The dichroic mirror passes a substantial amount of green light, on the order of five percent. The barrier filter does a much better job of removing the green light, attenuating the excitation wavelengths by about 5 orders of magnitude. The barrier filter is essential for making crisp, high-contrast images. | ||
| − | + | To provide collimated illumination in the sample plane, light from the illuminator is focused at the back focal point of the objective. | |
| − | + | ===Laser safety=== | |
| + | In this part of the lab, you will use a 5mW, 532 nm laser with focusing optics. You must attend the safety lecture before you work with the laser. See an instructor if you missed the lecture. Do not begin working with the laser until you thoroughly understand how to use it safely. | ||
| − | + | Stop working and ask an instructor immediately if you have any questions about working with lasers safely. | |
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | When you work with lasers, keep these laser safety best practices in mind: | |
| − | + | * Remove reflective clothing items and jewelry when working with lasers. | |
| + | * Use the lowest possible power during alignment or adjustment. | ||
| + | * Do not use reflective tools when the laser is on. | ||
| + | * Know the beam path at all times. | ||
| + | ** Use a stop to prevent uncontained beams. | ||
| + | ** Secure the stop so it will not move accidentally. | ||
| + | ** Keep your eyes out of the plane of the beam, which his normally a plane just above the optical table. | ||
| + | * Disable lasers when they are not in use. | ||
| + | * Use the correct safety goggles. | ||
| + | ** Always check the marking on the goggles to ensure they are appropriate for the laser you are working with. | ||
| + | ** Laser safety goggles are a last line of defense, not an excuse for unsafe practices. | ||
| + | * Do not operate a laser unless the flashing laser warning sign is on. | ||
| − | + | Some specific ways to work safely with the 20.309 fluorescence microscope: | |
| − | + | * Use an OD = 2 neutral density (ND) filter to attenuate the laser near the source. | |
| + | ** "OD" is an abbreviation for "optical density," which is log<sub>10</sub> of the power transmitted by the filter divided by the incident power. | ||
| + | ** "ND" means that the OD is equal for all wavelengths. | ||
| + | ** The ND filter attenuates the power of the laser by a factor of 10<sup>2</sup>, to about 50 μW, which is completely safe. | ||
| + | ** Mount the ND filter securely so there is no chance that it will get knocked out of place accidentally. | ||
| + | * The inverted microscope design directs laser light upward, toward the ceiling. Do not lean over the objective mount. Use a stop to prevent the beam from propagating above your microscope. | ||
| + | * Confine beams inside lens tubes. | ||
| + | * Disable the laser when it is unattended or stored by removing a battery, disconnecting the power source, or removing the key. | ||
| + | * Safety goggles for the laser in this lab should have an OD of at least 2 at 532 nm. | ||
| + | ** There are (at least) two different kinds of safety goggles in the lab that are appropriate for this lab. One has an OD of 2 at 532 nm and the other has an OD of 5. You can see the beam with the OD = 2 goggles, but the power is reduced to a safe level. This makes the OD 2 laser goggles very useful for making adjustments that must be done at full power. | ||
| − | [[Image:20. | + | ==Add laser illuminator, dichroic mirror, and barrier filter== |
| + | [[Image:20.309 130816 FluoMicroscope Annotated.png|thumb|right|400px|Layout of fluorescence microscope.]] | ||
| + | # Go over your design with one of the instructors before you start building. | ||
| + | # Use cage rods to construct a cage of appropriate size for the beam expander and excitation tube lens (L3, L4, and L5). | ||
| + | #* For maximum flexibility in positioning, removing, and reinstalling optics, use 3 cage rods instead of 4. Use a cage plate (CP02) for each lens. Mount the lens in a 1/2" lens tube (SM1L05) so it can be easily removed or installed. | ||
| + | # Add the two turning mirrors to the end of the cage. | ||
| + | #* Use a thick cage plate (CP02T) only to join 2 cages. (You will use a total of two CP02T plates in your microscope.) Don't use CP02T to mount lenses — there are not enough of them in the lab to use this way. It is easy to tell the difference. CP02T has 8 set screws and is roughly twice as thick as the CP02; CP02 has 4 set screws. | ||
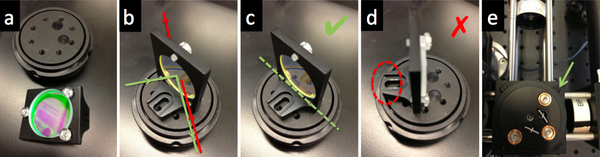
| + | # Use a cube optic mount (B5C) to mount dichroic mirror DM on a kinematic platform (B4C) . Place the kinematic platform in the cage cube (C6W). | ||
| + | #* The first surface of the dichroic should face the laser. | ||
| + | #** Some dichroics have an arrow indicating the first surface. If not, to ascertain which surface has the coating, watch the reflection of a corner of a piece of lens paper as it touches the mirror. On the first surface, the corner and its reflection will appear to touch. Held the other way, the corner and its image will appear a few millimeters apart. | ||
| + | #* Mount the dichroic so that the first surface lies on exactly a diameter of the B5C mount. | ||
| + | #* The mounting bracket should not stand in the way of the rotation of the kinematic stage. | ||
| + | #* Use the clear plastic #4/40 screws affix the dichroic mirror without deforming nor scratching its surface. | ||
| + | #* Hold the screwdriver at the tip to avoid slipping and scratching the dichroic. The screwdriver will instantly scratch the dichroic. | ||
| + | #* [[Image:20.309 130816 DichroicMirrorMount2.png|center|thumb|600px|Dichroic mirror mount]] | ||
| + | #* Ask an instructor for help if you need to clean a dichroic mirror or barrier filter. Dichroics have delicate, exposed coatings and must be cleaned with extra care. | ||
| + | # Use four black, plastic #4/40 screws to hold the cube optic mount (B5C) on the cage cube (C6W). | ||
| + | #* Tighten the screws enough so that the cube optic mount holds its adjustment, but can still be rotated. | ||
| + | #* Make sure to block the (small percentage of) excitation laser light that will be transmitted through the dichroic mirror with some black lab tape affixed to the B5C cube optic mount. | ||
| − | === | + | ===Align the laser illumination path=== |
| − | + | # Remove L3, L4, L5, and the objective lens L1. | |
| − | * | + | # Mount the laser. |
| − | * | + | # Insert a neutral density filter (ND filter) between the laser and M2 to reduce the laser power to a safe level for adjustment. |
| − | * | + | #* Remove jewelry. Turn of the laser before using reflective tools. |
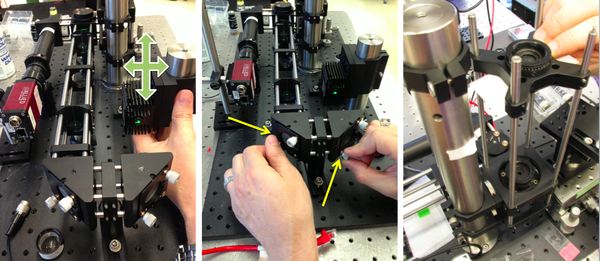
| + | # Turn on the laser. Use a beam stop until the laser position is set. Adjust the laser position so that the laser shines near the middle of M2. | ||
| + | #* You can use a DG10 mounted frosted-glass alignment disk with a pinhole to best center the laser beam onto M2. | ||
| + | # Adjust M2 and M3 to center the laser light in the cage. | ||
| + | #* Use two CPA1 alignment targets to gauge beam alignment. | ||
| + | #* [[Image:20.309 130816 AlignPinholes 4.png|center|thumb|600px|Using pinholes and irises to optimize initial microscope alignment. Notice that this person is breaking one of the laser safety rules. Which one?]] | ||
| + | # Adjust the dichroic mirror DM so that the beam will enter the middle of the objective lens. | ||
| + | #* Use a DG10 mounted frosted-glass alignment disk with a pinhole to show the optical center of the vertical beam path. | ||
| + | #* Verify that the centered beam is perpendicular to the floor. If the beam is at an angle, verify that the dichroic mirror DM is mounted on a diameter of the rotating mount. | ||
| + | # Insert the beam expander lenses, L3 and L4. Adjust the separation between L3 and L4 to achieve a collimated beam. | ||
| + | #* It may be necessary to make small adjustments to M2 and M3 to recenter the beam. | ||
| + | # Replace the excitation tube lens L5 and the objective lens L1. Adjust the position of L5 for best beam collimation (you can try to focus it far away). | ||
| + | #* The product of beam divergence and diameter is constant. L5 and L1 shrink the beam, causing increased divergence. The beam emerging from the objective will likely appear to grow in size as it propagates, even when the lenses are in their optimal positions. | ||
| + | #* It may be necessary to make small adjustments to M2 and M3 to recenter the beam. | ||
| + | # Use a fluorescence reference slide to center the field of view and to optimize the uniformity of illumination. | ||
| + | #* Make sure to use an ND filter when you use the fluorescence reference slide. The slide bleaches quickly at high intensities and you will not get a good image. | ||
| + | #* Put a barrier filter in the afocal part of the imaging path. | ||
| + | #* Center the camera's FOV in the objective's FOV. | ||
| + | #* If the laser light emerges from the objective at an angle, double-check your alignment and dichroic mounting. | ||
| + | #* The barrier filter has delicate, exposed metal coatings. Ask an instructor for help if you need to clean it. | ||
| − | ==== | + | ==Immunofluorescent staining== |
| + | In this part of the lab, you will investigate how a drug called cytochalasin D (CytoD) affects the structural properties of mammalian cells. Immunofluorescent staining will be used to make the actin cytoskeleton of epithelial cells visible. You will compare the structure of cells that have been treated with CytoD with that of untreated cells. | ||
| − | + | The mushroom toxin phalloidin binds to polymerized filamentous actin (F-actin) much more tightly than to actin monomers (G-actin), and stabilizes actin filaments by preventing their depolymerization. You will take advantage of this strong affinity between phalloidin and F-actin to image the cell's actin cytoskeleton. Phalloidin conjugated with the fluorescent dye Alexa Fluor 568 will be introduced in 3T3 mouse fibroblast cells. | |
| − | + | By comparing Alexa Fluor phalloidin-stained untreated cells and CytoD-treated cells, you will observe and quantify the effect CytoD has on the actin stress fiber network. Cytochalasin D is also a mycotoxin, but contrary to phalloidin, it poisons the cell by strongly inhibiting actin polymerization. | |
| − | ==== | + | {|class="wikitable" |
| + | |width="200"|[[File: 20.309 130904 PhalloidinActin.png|200px]] | ||
| + | |width="250"|[[File: Alexa568.jpg|250px]] | ||
| + | |- | ||
| + | |Example of a NIH 3T3 fibroblast cell labeled with rhodamine phalloidin | ||
| + | |Excitation and emission spectra for Alexa Fluor 568 | ||
| + | |} | ||
| + | {{Template:Safety Warning|message=Wear gloves when you are handling biological samples.}} | ||
| − | + | ===Procedures for fixing and labeling cells=== | |
| − | + | ||
| − | + | ||
| − | + | [[File: 3T3.PNG|thumb|200px|3T3 Swiss Albino]] | |
| + | You will be given two plates of cells: | ||
| + | * one dish will be left untreated, and then fixed and labeled with phalloidin; | ||
| + | * the second dish will be first treated with CytoD, and then fixed and labeled with phalloidin. | ||
| − | + | A key technique to keep in mind when working with live cells - to avoid shocking them with "cold" at 20°C - is to be sure that any solutions you add are pre-warmed to 37°C. We will keep a warm-water bath running for this purpose, in which we will keep the various media. | |
| − | + | ||
| − | + | ||
| − | + | You are provided with 3T3 cells, which were prepared as follows: | |
| + | Cells were cultured at 37°C in 5% CO<math>_2</math> in standard T75 flasks in a medium referred to as DMEM++. This consists of Dulbecco's Modified Eagle Medium (DMEM - Invitrogen) supplemented with 10% fetal bovine serum (FBS - Invitrogen), 1% of the antibiotic penicillin-streptomycin (Invitrogen), 1% non-essential amino acids, and 1% glutamine. The day prior to the fluorescence imaging, cells were plated on 35 mm glass-bottom cell culture dishes (MatTek, equipped with coverslip suited for optical microscopy studies). | ||
| − | + | '''Please read through the protocol for the two dishes below. Think about what you need to do so you can handle the dishes in parallel and save yourself some time.''' | |
| − | + | Below is the protocol to stain 3T3 cells with Alexa Fluor 568 phalloidin: | |
| + | * It is optimal for your cells to be ~60% confluent. If cells are too crowded, they will not stretch properly and show their beautiful actin filaments. Thus, you'll want to image cells that are stretched out and not overlapping much. Note also that these cells remain alive until the addition of formaldehyde, therefore requiring that any buffer/media added be pre-warmed. | ||
| − | + | # Pre-warm 3.7% formaldehyde solution and phosphate buffered saline at pH 7.4 (PBS) in a 37°C water bath if available. Keep the formaldehyde wrapped in foil to protect from light. | |
| + | # Retrieve an aliquot of cytochalasin D from the freezer and warm to 37°C. | ||
| − | + | <u>'''Dish 1--CytoD Treated Cells'''</u> | |
| + | * Prepare only ONE dish to be treated with cytochalasin D (CytoD). The other dish will be an untreated control. | ||
| + | # Remove the medium with a pipette and wash ONE dish 2X with 2 mL of pre-warmed PBS. Pipet into the dish gently to avoid washing away cells. | ||
| + | # Add 1 mL of the pre-warmed 10 μM CytoD solution to the same cell culture dish and incubate at 37°C for 20 minutes (''and not a minute longer!''). Afterwards, wash 2X with PBS. | ||
| − | < | + | <u>'''Dish 1 (CytoD) & Dish 2 (Untreated) Stained in Parallel'''</u> |
| − | + | # Remove the medium from the untreated dish (Dish 2) with a pipette and wash 2X with 2 mL of pre-warmed PBS. Pipet gently to avoid washing away cells. This assumes you've already washed your Cyto D plate (Dish 1). | |
| − | + | # Carefully pipet 400 μL of 3.7% formaldehyde solution onto the cells in the central glass region of each dish and incubate for 10 minutes at room temperature. This "fixes" the cells, ''i.e.'' cross-links the intracellular proteins and freezes the cell structure. | |
| + | # Wash each dish 3X with 1.5 mL PBS (note that this PBS solution no longer needs to be pre-warmed as the cells are dead). | ||
| + | # Extract each dish with 1.5 mL 0.1% Triton X-100 (a detergent) for 3-5 minutes. (Extraction refers to the partially dissolution of the plasma membrane of the cell.) | ||
| + | # Wash the cells 2X with 1.5 mL PBS. | ||
| + | # Incubate the fixed cells with 1.5 mL 1% BSA in PBS for 20 minutes. (BSA blocks the nonspecific binding sites.) | ||
| + | # Wash each dish 2X with PBS. | ||
| + | # Add 200 μL of Alexa Fluor 568 phalloidin solution (pre-mixed in methanol and PBS) to each dish. Carefully pipet this just onto the center of the dish, cover with aluminum foil, and incubate for 45 minutes at room temperature. | ||
| + | # Wash 3X with PBS. | ||
| + | # You can now store the sample at +4°C (regular refrigerator) in PBS for a few days, wrapped in parafilm and foil. | ||
| − | + | ==Fluorescence imaging== | |
| + | [[Image:20.309_130813_FluoExampleImages.png|thumb|Example images and histogram. Top: fluorescent beads; center: reference slide, bottom: image histogram.]] | ||
| − | + | In this part of the lab, you will make images of fluorescent microspheres plus your dishes of cells, and correct them for nonuniform illumination. In order to do the correction, you will need a reference image and dark images in addition to the image of the sample. See [[Flat-field correction|this page]] for more information about flat-field correction. | |
| − | + | ||
| − | + | * Reference Images: Take the reference image as close as possible in time to the sample images. Don't make any adjustments to your microscope between capturing the reference image and the sample image. For example, every time you move a mirror or re-align the laser, you will change the illumination profile and you must take a new reference. Adjusting the camera exposure and gain between recording the reference and sample images is okay. | |
| − | + | * Dark images: Each time you record an image (reference or sample), make sure to take a corresponding dark image using the EXACT SAME camera settings (''i.e. use the exact same Exposure Time and Gain settings you had chosen for your reference/sample image''). This is the only valid way to subtract the correct dark value from your reference/sample image. Use 12-bit imaging mode to get the best results. | |
| − | + | * Saving: Remember to save your images in a format that preserves all 12 bits. We recommend using the MATLAB <tt>save</tt> command to save data in a .mat file so you can reanalyze it later if necessary. You could also save in an image file format with <tt>imwrite</tt>. Convert the image to 16-bit, unsigned integer format with the correct range before saving. Read [[Optical Microscopy Part 1: Brightfield Microscopy#MATLABsaving|this section of Part 1]] if you need to refresh your memory. | |
| − | + | ||
| − | + | ===Take images=== | |
| − | + | # Record a reference image | |
| + | #* Put in an ND filter and take an image of the reference slide with the 40X objective. | ||
| + | #* Turn off the laser and record a dark image (without changing any camera settings!) | ||
| + | #* Use MATLAB to generate a histogram of to be certain that the image is exposed correctly. | ||
| + | # Remove the ND filter and image the samples: | ||
| + | #* 3.26 μm beads and a corresponding dark image | ||
| + | #* Fixed and stained untreated cells and a corresponding dark image | ||
| + | #* Fixed and stained cells treated with CytoD and a corresponding dark image | ||
| − | + | * For each of these samples remember to check the exposure, and to take a corresponding dark image | |
| − | + | * Visualizing the actin cytoskeleton under your 20.309 microscope will require skills. Since actin filaments and stress fibers are nanometer-scale objects, they are much dimmer than fluorescent beads or the dye solution - care must be taken to get good images of the cytoskeleton. You may need to cover the microscope to reduce room light contamination. You may find it helpful to use your brightfield microscope to find your cells before turning on your laser illumination, so as to minimize photobleaching of the fluorescent dye. | |
| − | + | * Adjust the gain (to zero) and exposure of the camera to get the best picture. | |
| − | + | ||
| − | + | ||
| − | + | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | + | [[Image:Fluor CHO 40x.png|thumb|Example image of CHO cells whose actin network is labeled with Alexa Fluor phalloidin]] | |
| − | == | + | ==Flat field correction== |
| + | # Perform flat-field correction on the images. | ||
| + | #* Divide the image by a normalized version of your reference image minus the dark image (see [[Flat-field correction|this page]] for more detail). | ||
| + | # '''Include the original, reference, and flat-field corrected images in your lab report.''' | ||
| − | + | ==Report requirements== | |
| − | + | ||
| − | + | ||
| − | + | ||
| − | === | + | ===General guidelines=== |
| + | {{:Microscopy report general guidelines}} | ||
| − | + | ===Part 2 report outline=== | |
| − | + | ||
| − | + | ||
| − | + | {{:Optical Microscopy: Part 2 Report Outline}} | |
| − | + | {{:Optical microscopy lab wiki pages}} | |
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
{{Template:20.309 bottom}} | {{Template:20.309 bottom}} | ||
Latest revision as of 22:06, 14 August 2017
|
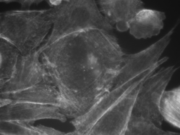
3T3 cells. Actin stained with Alexa Fluor 568 Phalloidin. |
Same cell type incubated with cytochalasin D, an inhibitor of actin polymerization, prior to fixing and stained. |
|---|
Overview
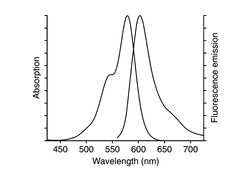
In this part of the lab, you will add epifluorescence imaging capability to your microscope, make images of fluorescent samples, and process the images to correct artifacts caused by nonuniform illumination. The samples you will image include fluorescent microspheres of several sizes and cells stained with a fluorescent dye. A typical spectrum is shown on the right. The excitation light source is a 5 mW diode laser with a nominal wavelength of 532 nm — a striking, brilliant green. Emission is in the red/orange range. To make the correction for nonuniform illumination, you will also make images of a uniform fluorescence reference slide and a dark image with the illuminator turned off.
As shown in the block diagram, the major components required for fluorescence imaging are an illuminator (laser, L3, L4, and L5), dichroic mirror (DM), and emission filter (BF). The illuminator provides light in the appropriate wavelength range to excite the fluorescent molecules in the sample. Fluorescence microscopes that use broadand light sources such as arc lamps require an additional filter called an excitation filter to limit the wavelengths in the illumination to the proper range. Because lasers emit light in a very narrow range of wavelengths, an excitation filter is unnecessary.
Excitation light comes from beneath the sample, through the objective lens. A dichroic mirror directs the excitation toward the objective and sample. The mirror must reflect wavelengths in the excitation range and pass the longer wavelengths of the emitted fluorescence. In fluorescence imaging, illumination intensity is typically 5 or 6 orders of magnitude greater than emitted fluorescence, so it is crucial to filter out excitation photons as completely as possible. The dichroic mirror passes a substantial amount of green light, on the order of five percent. The barrier filter does a much better job of removing the green light, attenuating the excitation wavelengths by about 5 orders of magnitude. The barrier filter is essential for making crisp, high-contrast images.
To provide collimated illumination in the sample plane, light from the illuminator is focused at the back focal point of the objective.
Laser safety
In this part of the lab, you will use a 5mW, 532 nm laser with focusing optics. You must attend the safety lecture before you work with the laser. See an instructor if you missed the lecture. Do not begin working with the laser until you thoroughly understand how to use it safely.
Stop working and ask an instructor immediately if you have any questions about working with lasers safely.
When you work with lasers, keep these laser safety best practices in mind:
- Remove reflective clothing items and jewelry when working with lasers.
- Use the lowest possible power during alignment or adjustment.
- Do not use reflective tools when the laser is on.
- Know the beam path at all times.
- Use a stop to prevent uncontained beams.
- Secure the stop so it will not move accidentally.
- Keep your eyes out of the plane of the beam, which his normally a plane just above the optical table.
- Disable lasers when they are not in use.
- Use the correct safety goggles.
- Always check the marking on the goggles to ensure they are appropriate for the laser you are working with.
- Laser safety goggles are a last line of defense, not an excuse for unsafe practices.
- Do not operate a laser unless the flashing laser warning sign is on.
Some specific ways to work safely with the 20.309 fluorescence microscope:
- Use an OD = 2 neutral density (ND) filter to attenuate the laser near the source.
- "OD" is an abbreviation for "optical density," which is log10 of the power transmitted by the filter divided by the incident power.
- "ND" means that the OD is equal for all wavelengths.
- The ND filter attenuates the power of the laser by a factor of 102, to about 50 μW, which is completely safe.
- Mount the ND filter securely so there is no chance that it will get knocked out of place accidentally.
- The inverted microscope design directs laser light upward, toward the ceiling. Do not lean over the objective mount. Use a stop to prevent the beam from propagating above your microscope.
- Confine beams inside lens tubes.
- Disable the laser when it is unattended or stored by removing a battery, disconnecting the power source, or removing the key.
- Safety goggles for the laser in this lab should have an OD of at least 2 at 532 nm.
- There are (at least) two different kinds of safety goggles in the lab that are appropriate for this lab. One has an OD of 2 at 532 nm and the other has an OD of 5. You can see the beam with the OD = 2 goggles, but the power is reduced to a safe level. This makes the OD 2 laser goggles very useful for making adjustments that must be done at full power.
Add laser illuminator, dichroic mirror, and barrier filter
- Go over your design with one of the instructors before you start building.
- Use cage rods to construct a cage of appropriate size for the beam expander and excitation tube lens (L3, L4, and L5).
- For maximum flexibility in positioning, removing, and reinstalling optics, use 3 cage rods instead of 4. Use a cage plate (CP02) for each lens. Mount the lens in a 1/2" lens tube (SM1L05) so it can be easily removed or installed.
- Add the two turning mirrors to the end of the cage.
- Use a thick cage plate (CP02T) only to join 2 cages. (You will use a total of two CP02T plates in your microscope.) Don't use CP02T to mount lenses — there are not enough of them in the lab to use this way. It is easy to tell the difference. CP02T has 8 set screws and is roughly twice as thick as the CP02; CP02 has 4 set screws.
- Use a cube optic mount (B5C) to mount dichroic mirror DM on a kinematic platform (B4C) . Place the kinematic platform in the cage cube (C6W).
- The first surface of the dichroic should face the laser.
- Some dichroics have an arrow indicating the first surface. If not, to ascertain which surface has the coating, watch the reflection of a corner of a piece of lens paper as it touches the mirror. On the first surface, the corner and its reflection will appear to touch. Held the other way, the corner and its image will appear a few millimeters apart.
- Mount the dichroic so that the first surface lies on exactly a diameter of the B5C mount.
- The mounting bracket should not stand in the way of the rotation of the kinematic stage.
- Use the clear plastic #4/40 screws affix the dichroic mirror without deforming nor scratching its surface.
- Hold the screwdriver at the tip to avoid slipping and scratching the dichroic. The screwdriver will instantly scratch the dichroic.
- Ask an instructor for help if you need to clean a dichroic mirror or barrier filter. Dichroics have delicate, exposed coatings and must be cleaned with extra care.
- The first surface of the dichroic should face the laser.
- Use four black, plastic #4/40 screws to hold the cube optic mount (B5C) on the cage cube (C6W).
- Tighten the screws enough so that the cube optic mount holds its adjustment, but can still be rotated.
- Make sure to block the (small percentage of) excitation laser light that will be transmitted through the dichroic mirror with some black lab tape affixed to the B5C cube optic mount.
Align the laser illumination path
- Remove L3, L4, L5, and the objective lens L1.
- Mount the laser.
- Insert a neutral density filter (ND filter) between the laser and M2 to reduce the laser power to a safe level for adjustment.
- Remove jewelry. Turn of the laser before using reflective tools.
- Turn on the laser. Use a beam stop until the laser position is set. Adjust the laser position so that the laser shines near the middle of M2.
- You can use a DG10 mounted frosted-glass alignment disk with a pinhole to best center the laser beam onto M2.
- Adjust M2 and M3 to center the laser light in the cage.
- Use two CPA1 alignment targets to gauge beam alignment.
- Adjust the dichroic mirror DM so that the beam will enter the middle of the objective lens.
- Use a DG10 mounted frosted-glass alignment disk with a pinhole to show the optical center of the vertical beam path.
- Verify that the centered beam is perpendicular to the floor. If the beam is at an angle, verify that the dichroic mirror DM is mounted on a diameter of the rotating mount.
- Insert the beam expander lenses, L3 and L4. Adjust the separation between L3 and L4 to achieve a collimated beam.
- It may be necessary to make small adjustments to M2 and M3 to recenter the beam.
- Replace the excitation tube lens L5 and the objective lens L1. Adjust the position of L5 for best beam collimation (you can try to focus it far away).
- The product of beam divergence and diameter is constant. L5 and L1 shrink the beam, causing increased divergence. The beam emerging from the objective will likely appear to grow in size as it propagates, even when the lenses are in their optimal positions.
- It may be necessary to make small adjustments to M2 and M3 to recenter the beam.
- Use a fluorescence reference slide to center the field of view and to optimize the uniformity of illumination.
- Make sure to use an ND filter when you use the fluorescence reference slide. The slide bleaches quickly at high intensities and you will not get a good image.
- Put a barrier filter in the afocal part of the imaging path.
- Center the camera's FOV in the objective's FOV.
- If the laser light emerges from the objective at an angle, double-check your alignment and dichroic mounting.
- The barrier filter has delicate, exposed metal coatings. Ask an instructor for help if you need to clean it.
Immunofluorescent staining
In this part of the lab, you will investigate how a drug called cytochalasin D (CytoD) affects the structural properties of mammalian cells. Immunofluorescent staining will be used to make the actin cytoskeleton of epithelial cells visible. You will compare the structure of cells that have been treated with CytoD with that of untreated cells.
The mushroom toxin phalloidin binds to polymerized filamentous actin (F-actin) much more tightly than to actin monomers (G-actin), and stabilizes actin filaments by preventing their depolymerization. You will take advantage of this strong affinity between phalloidin and F-actin to image the cell's actin cytoskeleton. Phalloidin conjugated with the fluorescent dye Alexa Fluor 568 will be introduced in 3T3 mouse fibroblast cells.
By comparing Alexa Fluor phalloidin-stained untreated cells and CytoD-treated cells, you will observe and quantify the effect CytoD has on the actin stress fiber network. Cytochalasin D is also a mycotoxin, but contrary to phalloidin, it poisons the cell by strongly inhibiting actin polymerization.
|
|
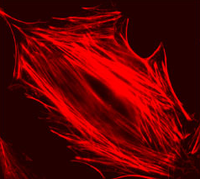
| Example of a NIH 3T3 fibroblast cell labeled with rhodamine phalloidin | Excitation and emission spectra for Alexa Fluor 568 |
| |
Wear gloves when you are handling biological samples. |
Procedures for fixing and labeling cells
You will be given two plates of cells:
- one dish will be left untreated, and then fixed and labeled with phalloidin;
- the second dish will be first treated with CytoD, and then fixed and labeled with phalloidin.
A key technique to keep in mind when working with live cells - to avoid shocking them with "cold" at 20°C - is to be sure that any solutions you add are pre-warmed to 37°C. We will keep a warm-water bath running for this purpose, in which we will keep the various media.
You are provided with 3T3 cells, which were prepared as follows: Cells were cultured at 37°C in 5% CO$ _2 $ in standard T75 flasks in a medium referred to as DMEM++. This consists of Dulbecco's Modified Eagle Medium (DMEM - Invitrogen) supplemented with 10% fetal bovine serum (FBS - Invitrogen), 1% of the antibiotic penicillin-streptomycin (Invitrogen), 1% non-essential amino acids, and 1% glutamine. The day prior to the fluorescence imaging, cells were plated on 35 mm glass-bottom cell culture dishes (MatTek, equipped with coverslip suited for optical microscopy studies).
Please read through the protocol for the two dishes below. Think about what you need to do so you can handle the dishes in parallel and save yourself some time.
Below is the protocol to stain 3T3 cells with Alexa Fluor 568 phalloidin:
- It is optimal for your cells to be ~60% confluent. If cells are too crowded, they will not stretch properly and show their beautiful actin filaments. Thus, you'll want to image cells that are stretched out and not overlapping much. Note also that these cells remain alive until the addition of formaldehyde, therefore requiring that any buffer/media added be pre-warmed.
- Pre-warm 3.7% formaldehyde solution and phosphate buffered saline at pH 7.4 (PBS) in a 37°C water bath if available. Keep the formaldehyde wrapped in foil to protect from light.
- Retrieve an aliquot of cytochalasin D from the freezer and warm to 37°C.
Dish 1--CytoD Treated Cells
- Prepare only ONE dish to be treated with cytochalasin D (CytoD). The other dish will be an untreated control.
- Remove the medium with a pipette and wash ONE dish 2X with 2 mL of pre-warmed PBS. Pipet into the dish gently to avoid washing away cells.
- Add 1 mL of the pre-warmed 10 μM CytoD solution to the same cell culture dish and incubate at 37°C for 20 minutes (and not a minute longer!). Afterwards, wash 2X with PBS.
Dish 1 (CytoD) & Dish 2 (Untreated) Stained in Parallel
- Remove the medium from the untreated dish (Dish 2) with a pipette and wash 2X with 2 mL of pre-warmed PBS. Pipet gently to avoid washing away cells. This assumes you've already washed your Cyto D plate (Dish 1).
- Carefully pipet 400 μL of 3.7% formaldehyde solution onto the cells in the central glass region of each dish and incubate for 10 minutes at room temperature. This "fixes" the cells, i.e. cross-links the intracellular proteins and freezes the cell structure.
- Wash each dish 3X with 1.5 mL PBS (note that this PBS solution no longer needs to be pre-warmed as the cells are dead).
- Extract each dish with 1.5 mL 0.1% Triton X-100 (a detergent) for 3-5 minutes. (Extraction refers to the partially dissolution of the plasma membrane of the cell.)
- Wash the cells 2X with 1.5 mL PBS.
- Incubate the fixed cells with 1.5 mL 1% BSA in PBS for 20 minutes. (BSA blocks the nonspecific binding sites.)
- Wash each dish 2X with PBS.
- Add 200 μL of Alexa Fluor 568 phalloidin solution (pre-mixed in methanol and PBS) to each dish. Carefully pipet this just onto the center of the dish, cover with aluminum foil, and incubate for 45 minutes at room temperature.
- Wash 3X with PBS.
- You can now store the sample at +4°C (regular refrigerator) in PBS for a few days, wrapped in parafilm and foil.
Fluorescence imaging
In this part of the lab, you will make images of fluorescent microspheres plus your dishes of cells, and correct them for nonuniform illumination. In order to do the correction, you will need a reference image and dark images in addition to the image of the sample. See this page for more information about flat-field correction.
- Reference Images: Take the reference image as close as possible in time to the sample images. Don't make any adjustments to your microscope between capturing the reference image and the sample image. For example, every time you move a mirror or re-align the laser, you will change the illumination profile and you must take a new reference. Adjusting the camera exposure and gain between recording the reference and sample images is okay.
- Dark images: Each time you record an image (reference or sample), make sure to take a corresponding dark image using the EXACT SAME camera settings (i.e. use the exact same Exposure Time and Gain settings you had chosen for your reference/sample image). This is the only valid way to subtract the correct dark value from your reference/sample image. Use 12-bit imaging mode to get the best results.
- Saving: Remember to save your images in a format that preserves all 12 bits. We recommend using the MATLAB save command to save data in a .mat file so you can reanalyze it later if necessary. You could also save in an image file format with imwrite. Convert the image to 16-bit, unsigned integer format with the correct range before saving. Read this section of Part 1 if you need to refresh your memory.
Take images
- Record a reference image
- Put in an ND filter and take an image of the reference slide with the 40X objective.
- Turn off the laser and record a dark image (without changing any camera settings!)
- Use MATLAB to generate a histogram of to be certain that the image is exposed correctly.
- Remove the ND filter and image the samples:
- 3.26 μm beads and a corresponding dark image
- Fixed and stained untreated cells and a corresponding dark image
- Fixed and stained cells treated with CytoD and a corresponding dark image
- For each of these samples remember to check the exposure, and to take a corresponding dark image
- Visualizing the actin cytoskeleton under your 20.309 microscope will require skills. Since actin filaments and stress fibers are nanometer-scale objects, they are much dimmer than fluorescent beads or the dye solution - care must be taken to get good images of the cytoskeleton. You may need to cover the microscope to reduce room light contamination. You may find it helpful to use your brightfield microscope to find your cells before turning on your laser illumination, so as to minimize photobleaching of the fluorescent dye.
- Adjust the gain (to zero) and exposure of the camera to get the best picture.
Flat field correction
- Perform flat-field correction on the images.
- Divide the image by a normalized version of your reference image minus the dark image (see this page for more detail).
- Include the original, reference, and flat-field corrected images in your lab report.
Report requirements
General guidelines
- Each part of the microscopy lab requires its own submission. Only the final report will include all 3 sections. You may revise any part of your report until the final deadline. Update the apparatus section to reflect any changes you make during the course of the lab.
- On each due date, one member of your group should submit a single PDF file to Stellar in advance of the deadline. The filename should consist of the last names of all group members, CamelCased, in alphabetical order, followed by a hyphen, the name of the assignment, with a .pdf extension. Example: CrickFranklinWatson-MicroscopyPart1.pdf.
- Use the outline below to break your report into sections.
- Keep your lab report short. Use bullet points, tables, and other organizational tools when appropriate. Brevity is not an excuse for imprecise, incomplete, or unclear communication.
- Be selective in what images you include in your report. Where images are required, include one or a few that convey the character of the dataset. Size images appropriately (does it really need to take up half a page?). At the same time, ensure plots images are readable. A good trick for reducing plot size while maintaining clarity is to adjust the font size and line values so the plot remains clear even when it is small. It helps to save images and plots in an uncompressed format; import and resize them in a way that retains the full quality; and create your final PDF file with settings that do not compress plots and pictures.
- An outstanding error discussion is an essential element of a top-notch report.
- Include any computer code you developed in an appendix at the end of your report. Indicate the source of any code used that you obtained from outside sources.
- Refer to this page for more detail on how to write an excellent report, and to this page for an outline of the final microscopy report.
Part 2 report outline
- Microscope documentation
- Include an updated block diagram of your microscope.
- Images
- Include a figure with an images of the 3.26 μm fluorescent microsphere samples, and the stained cell samples with and without Cyto-D.
- For each sample, create 1 figure with 5 panels.
- The panels of the figure should be: A) unprocessed image; B) reference image; C) dark image; D) flat-field corrected image; and E) histogram.
- In the caption, specify the exposure and gain settings. Each image should have a scale bar. State the dimension of the scale bar in the caption.
- For panel E, plot histograms of the unprocessed, dark, reference, and corrected image on the same set of axes. Plot log10( count ) on the vertical axis and intensity on the horizontal axis. Use a line plot instead of a bar chart for the histogram.
- Image profile
- For one reference, dark and cell image set, plot an intensity profile across the same diagonal. You may also use a bead image, along with it's unique reference and dark images. The intensity of your three images should be on the same scale, i.e., 0 to 65,535 or 0 to 1. Place all three profiles on a single set of axes for comparison. (Use the improfile command in MATLAB.)
- Include a figure with an images of the 3.26 μm fluorescent microsphere samples, and the stained cell samples with and without Cyto-D.
- Discussion
- How did your beam expander design affect your images?
- What differences did you observe between the cells with and without CytoD?
Optical microscopy lab
Code examples and simulations
- Converting Gaussian fit to Rayleigh resolution
- MATLAB: Estimating resolution from a PSF slide image
- Matlab: Scalebars
- Calculating MSD and Diffusion Coefficients