Difference between revisions of "20.109(F20):M2D2"
Noreen Lyell (Talk | contribs) (→Reagents) |
Noreen Lyell (Talk | contribs) (→Reagents) |
||
| Line 123: | Line 123: | ||
*Elution buffer: 100 mM HEPES (pH = 7.4), 500 mM NaCl, 250 mM imidazole | *Elution buffer: 100 mM HEPES (pH = 7.4), 500 mM NaCl, 250 mM imidazole | ||
*imidazole (from Sigma) | *imidazole (from Sigma) | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
==Navigation links== | ==Navigation links== | ||
Next day: [[20.109(F20):M2D3 | Assess purity and concentration of purified protein]]<br> | Next day: [[20.109(F20):M2D3 | Assess purity and concentration of purified protein]]<br> | ||
Previous day: [[20.109(F20):M2D1 | Complete in silico cloning of protein expression plasmid]] | Previous day: [[20.109(F20):M2D1 | Complete in silico cloning of protein expression plasmid]] | ||
Revision as of 13:07, 17 July 2020
Introduction
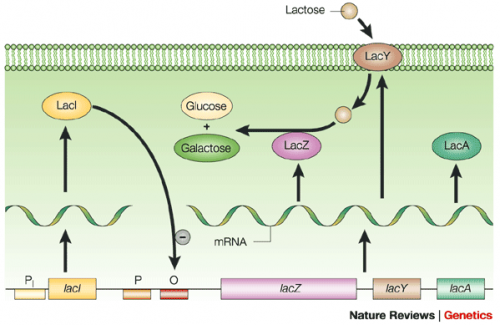
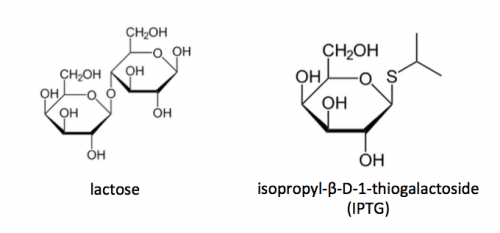
To induce production of PF3D7_1351100 protein from the expression plasmid that was 'cloned' in the previous laboratory session, a lactose-analogue isopropyl β-D-1-thiogalactopyranoside (IPTG) and arabinose was used to induce expression in Nico(DE3) E. coli bacterial cells. The use of IPTG to induce protein expression is based on the native lac operon used for lactose metabolism in bacterial cells.
The lac operon is composed of four genes: lacI, lacZ, lacY, and lacA. When lactose is absent, LacI (the protein encoded by lacI) binds to the operator sequence (O) upstream of lacZYA. In the presence of lactose, LacI and lactose form a complex which relieves repression of lacZYA transcription. LacZ is a β-galactosidase that cleaves lactose resulting in glucose and galactose. LacY, a β-galactoside permease, facilitates the transport of lactose across the cell membrane, and LacA, a β-galactoside transacetylase, transfers an acetyl group from acetyl-CoA to β-galactosides.The native lac operon is a powerful tool in engineering protein expression systems because it enables researchers to control gene expression using inducer molecules. The lacZYA genes are only expressed when lactose is present. If a gene of interest is cloned downstream of the operator sequence, the expression of this gene can be controlled by LacI repression and lactose derepression. To further control the system for protein expression, IPTG is used as a lactose-analog as it is not metabolized by the cells.
Today you will isolate the expressed PF3D7_1351100 protein from the bacterial cells. Remember that the PF3D7_1351100 gene sequence was synthesized using a gBlock and 6xHis-tag was added to the DNA sequence. The resultant protein is therefore His-tagged. Histidine has several interesting properties, notably its near-neutral pKa, and His-rich peptides are promiscuous binders, particularly to metals. (For example, histidine side chains help coordinate iron molecules in hemoglobin.)
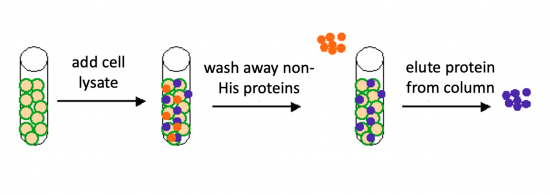
You will use a nickel-agarose resin to separate PF3D7_1351100 from the other proteins present in the bacteria. The His-tagged protein will preferentially bind to the nickel-coated beads, while proteins irrelevant to our purposes can be washed away using imidazole. Remember, the Nico(DE3) cells are not only producing the PF3D7_1351100 protein, but also the proteins needed for cellular function and survival. Because imidazole is also able to bind to nickel, washing the column with a low concentration solution will promote competition for binding between the imidazole and bound proteins. Proteins that are non-specifically bound will have a lower affinity for the nickel than imidazole and be washed from the column, whereas the 6xHis-tagged PF3D7_1351100 will remain adhered to the nickel-agarose resin.
Protocols
Part 1: Induce expression of PF3D7_1351100
To ensure the steps included below are clear, please watch the video tutorial (linked [[ | here]]). The steps are detailed below so you can follow along!
- Inoculated 5 mL of TB media containing 50 μg/mL kanamycin with a colony of Nico(DE3) cells transformed with pET-28b(+)_PF3D7_1351100.
- Incubated the culture overnight at 37 °C with shaking at 220 rpm.
- Dilute the overnight culture 1:100 in 100 mL of fresh TB media containing 50 μg/mL kanamycin.
- Incubate at 37 °C until the OD600 = 0.6 with shaking at 220 rpm.
- To induce PF3D7_1351100 protein expression, add IPTG to a final concentration of 1 mM.
- Incubate at overnight at room temperature with shaking at 100 rpm.
- To harvest the cells, centrifuge the culture at 4000 g for 15 min at 4 °C.
- Cell pellets were stored at -80 °C until used for purification.
In your laboratory notebook, complete the following:
- Calculate the volume of kanamycin stock that was added to the TB broth in Step #1. In Step #3.
- Concentration of kanamycin stock = 50 μg/mL.
- Calculate the volume of IPTG stock that was added to the TB broth in Step #5.
- Concentration of IPTG stock = 100 mM.
Part 2: Purify PF3D7_1351100 protein
To ensure the steps required for purifying the PF3D7_1351100 protein are clear, the Instructor will provide a live demonstration of this process. You should include notes regarding the procedure in your laboratory notebook!
Lyse Nico(DE3) cells expressing pET-28b(+)_PF3D7_1351100
- Retrieve the Nico(DE3) pET-28b(+)_PF3D7_1351100 cell pellet from the -80 °C freezer and leave it on your bench to thaw.
- Prepare the cell lysis buffer by combining the following reagents:
- 1 mL B-Per bacterial extraction reagent
- 2 μL lysozyme
- 2 μL DNAse I
- AEBSF to a final concentration of 1 mM
- Solubilize the cell pellet in 1 mL of cell lysis buffer and vortex to mix.
- Obtain a 3 mL aliquot of lysis buffer from the front laboratory bench.
- Calculate volume of imidazole needed in the lysis buffer such that the final concentration of imidazole is 10 mM (stock concentration = 2.5 M).
- Resuspend each pellet completely in 1.5 mL of the lysis buffer with imidazole.
- Add 5μL of the lysonase to each cell suspension.
- Incubate at room temperature for 10 min on a nutator.
- A sonicator will be used to lyse the cells. In a process called soniporation, sound energy is produced via a sonicator and exerted through a probe that rests in the sample liquid. This results in lysis of the cell membrane which releases the cell contents. The Instructor will lyse your sample with the sonicator in the 4 °C cold room using the following settings: 50% duty cycle for 4 min at power 4.
- Because sonication is more effective when completed with larger volumes, you will combine your samples with those of two other teams. The Instructor will inform you which team samples should be combined.
- Divide cell lysate evenly amongst the teams combined for sonication (~1500 μL per tube) and centrifuge at 12,500g for 30 min.
Prepare Ni-NTA affinity column
- Obtain a 500 μL aliquot of 50% slurry (Ni-NTA resin) and mix the slurry by inverting the tube several times.
- The slurry is the Ni-NTA column matrix!
- Centrifuge the slurry for 30 sec then remove the supernatent.
- To wash the slurry, add 500 μL of 1X PBS and invert the tube 3 times.
- Add the slurry to the column and allow the 1X PBS to run through the column.
- Be sure a beaker is placed under the column to collect the waste!
- When the PBS has flowed through, cap the bottom and the top of the column until you are ready to add the cell lysate.
Purify PF3D7_1351100
- Retrieve your cell pellets from the centrifuge and transfer the supernatent to a fresh 2 mL tube.
- Label the tube containing the cell pellet and give it to the teaching faculty!
- Note: It is VERY important that you collect fraction samples as indicated in Step 2, Step 8, & Step 9!
- Transfer 30 μL into labeled 1.5 mL eppendorf tubes and give the aliquots to the teaching faculty. Also label and save the pellet at this step.
- You will use this aliquot to examine protein yield prior to purification using polyacrylamide gel electrophoresis (PAGE) on a later date. At that time, the teaching faculty will provide you with a bacterial pellet from uninduced bacteria to compare.
- Note: Why would you want to compare induced to uninduced bacteria?
- Add 10 μL of the SnapTag substrate to the supernatant.
- Next add DTT to the supernatant at a final concentration of 1 mM.
- Note: the SnapTag substrate / buffer reaction allows the TDP43 protein to be labeled with a 647nm fluorophore at the SNAP sequence incorporated into the protein construct.
- Transfer the supernatant to the slurry prepared in Part 3.
- Note: the slurry consists of a nickel resin that will bind His-tagged proteins.
- Use aluminum foil to wrap the tube such that the supernatent / slurry are protected from light.
- Incubate the supernatent / slurry for 2 hr (or until 4:30p, be sure to record the exact incubation time in your notebook!) in the 4 °C cooler on a nutator.
- What is happening during this incubation? Hint: consider the purpose of the SnapTag and that of the nickel resin slurry.
- Following the incubation, reclamp the column in the ring stand to collect the flowthrough.
- Prepare a 1.5 mL tube under the column to collect the flow through (liquid that drips from the column). Only remove the bottom cap when you are ready to collect all flow through.
- To wash the slurry in the column, add 2.5 mL of 1X PBS containing 10 mM imidazole.
- Collect ~250 μL of the first wash flowthrough (in a well-labeled 1.5 mL tube), the rest of the flowthrough is waste and will be collected in the beaker under the column.
- What is in the flowthrough? What is attached to the nickel resin slurry?
- Repeat the 2.5 mL of 1X PBS with imidazole wash step a total of 3 times.
- Next, wash the slurry with 2 mL of HRV 3C buffer.
- Cap the bottom of the column.
- Note: It is very important that the column is capped before the next step, if not the protein will be lost in the waste beaker!
- In the remaining 1 mL of HRV 3C buffer, add 10 μL of HRV 3C protease. Then add the HRV 3C buffer / protease to the capped column.
- Resuspend the slurry in the HRV 3C buffer / protease by pipetting up and down with a P1000.
- Add a cap to the top of the column and wrap the column in aluminum foil.
- Incubate the slurry overnight at 4 °C on a rotator wheel.
- The next steps will be completed by the teaching faculty, but should be included in your methods!
- Following the overnight incubation, uncap the base of the column and collect all flow through.
- Resuspend the slurry in 250 μL 1X PBS and transfer to a fresh tube.
Concentrate purified PF3D7_1351100
blah
Reagents
- Terrific broth (TB) (from RPI)
- kanamycin (from Sigma)
- isopropyl β-d-1-thiogalactopyranoside (IPTG) (from Sigma)
- 2x B-Per bacterial protein extraction reagent (from Thermo Fisher)
- lysozyme (from Sigma)
- DNase I (from Sigma)
- 4-(2-aminoethyl)benzenesulfonyl fluoride (AEBSF) (from Sigma)
- phosphate saline buffer (PBS) (from VWR)
- Ni-NTA agarose (from Qiagen)
- Wash buffer: 100 mM HEPES (pH = 7.4), 500 mM NaCl, 10 mM imidazole
- Elution buffer: 100 mM HEPES (pH = 7.4), 500 mM NaCl, 250 mM imidazole
- imidazole (from Sigma)
Next day: Assess purity and concentration of purified protein