Difference between revisions of "20.109(S17):Confirm cell lines and practice tissue culture techniques (Day1)"
Noreen Lyell (Talk | contribs) (→Protocols) |
Noreen Lyell (Talk | contribs) (→Introduction) |
||
| Line 3: | Line 3: | ||
==Introduction== | ==Introduction== | ||
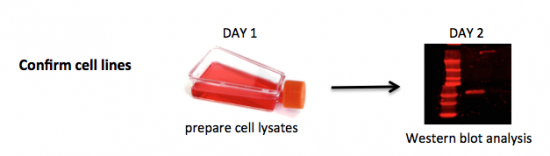
| + | To be confident of your findings related to DNA repair, you will first validate the cell lines you are using to assess the effect of drug treatment following DNA damage on NHEJ. It is important to confirm the cells you are using are indeed mutants in the BRCA2 protein to interpret the data you collect in this module. You will do this by completing a Western blot. A Western blot allows researchers to detect specific proteins within a mixed sample, such as cell lysate. We will use this protein analysis technique to assess BRCA2 levels in the wild-type DLD-1 and mutant DLD-1 BRCA2-/- (referred to as BRCA2-) cell lines. The wild-type or 'parental' DLD-1 cell line originated from a the colon of a male patient with colorectal adenocarcinoma Dukes Type C. Using recombinant adeno-associated virus gene editing technology, the BRCA2- mutant cell line was created via disruption of BRCA2 exon 11. | ||
| + | |||
| + | [[Image:Sp17 20.109 M2D1 confirm cells.png|thumb|550px|center|]] | ||
| + | |||
| + | Tissue culture was developed ~100 years ago as a means to study mammalian biology, and since that time we have learned a tremendous amount by observing the behavior of mammalian cells maintained in the laboratory. The term tissue culture was originally coined because researchers were doing exactly that, extracting tissue and letting it live in a dish for a short time. Today, most tissue culture experiments are done using cells rather than tissues. Much of what we know about cancer, heritable diseases, and the effects of the environment on human health has been derived from studies of cultured cells. | ||
| + | |||
| + | What types of cells do people study, and from where do they come? Cells acquired directly from animal tissue are called primary cells. They are difficult to culture, largely because primary cells in this context divide only a limited number of times. This limitation on the lifespan of cultured primary cells, called the Hayflick limit, is a problem because it requires a researcher to repeatedly remove tissues from animals to complete a study. Cell isolation processes can be quite labor-intensive, and also can complicate data analysis due to inherent animal-to-animal variation. To get around this problem, researchers study cells that are immortal, which means the cells are able to divide indefinitely. Though using immortal cells is preferable for many reasons, some inherent cell-to-cell variation still exists in such populations and the genetic changes that cause immortality may affect experimental outcomes. | ||
| + | |||
| + | The art of tissue culture lies in the ability to create conditions that are similar to what a cell would experience in an animal, namely 37°C and neutral pH. Blood nourishes the cells in an animal, and blood components are therfore used to feed cells in culture. Serum, the cell-free (and clotting-factor free) component of blood, contains many of the factors necessary to support the growth of cells outside the animal. Consequently, serum is frequently added to tissue culture medium, although serum-free media (also called chemically defined media) exist and support some types of cultured cells. | ||
| + | |||
| + | Cultured mammalian cells must grow in a germ-free environment and researchers using tissue culture must be skilled in sterile or asceptic technique. Bacteria double very quickly relative to mammalian cells. An average mammalian cell doubles about once per day whereas many common bacteria can double every 20 minutes under optimal conditions. Consequently, if you were to co-culture 100 mammalian cells and 1 bacteria together in a flask, within 24 hours you would have ~200 unhappy mammalian cells, and about 100 million happy bacteria! Needless to say, you would not find it very useful to continue to study the behavior of your mammalian cells under these conditions. | ||
| + | |||
| + | Today you will learn and practice asceptic technique to collect and count cells that were previously cultured by the teaching faculty. You will use the cells you collect to seed (or culture) cells for a survival assay that will assess the affect of drug treatment following DNA damage. To ensure you are well-prepared for your work in the tissue culture room, your instructor will provide a demonstration before you begin. Because the tissue culture room is not large enough to accommodate the entire class at once, we will be split into two groups today...and all subsequent days that involve tissue culture. Half of the class will begin in tissue culture with Part 1 and the other half of the class will begin by preparing previously seeded DLD-1 and BRCA2- cells for Western blot analysis. Your instructor will designate the groups. | ||
==Protocols== | ==Protocols== | ||
Revision as of 18:45, 1 February 2017
Contents
Introduction
To be confident of your findings related to DNA repair, you will first validate the cell lines you are using to assess the effect of drug treatment following DNA damage on NHEJ. It is important to confirm the cells you are using are indeed mutants in the BRCA2 protein to interpret the data you collect in this module. You will do this by completing a Western blot. A Western blot allows researchers to detect specific proteins within a mixed sample, such as cell lysate. We will use this protein analysis technique to assess BRCA2 levels in the wild-type DLD-1 and mutant DLD-1 BRCA2-/- (referred to as BRCA2-) cell lines. The wild-type or 'parental' DLD-1 cell line originated from a the colon of a male patient with colorectal adenocarcinoma Dukes Type C. Using recombinant adeno-associated virus gene editing technology, the BRCA2- mutant cell line was created via disruption of BRCA2 exon 11.
Tissue culture was developed ~100 years ago as a means to study mammalian biology, and since that time we have learned a tremendous amount by observing the behavior of mammalian cells maintained in the laboratory. The term tissue culture was originally coined because researchers were doing exactly that, extracting tissue and letting it live in a dish for a short time. Today, most tissue culture experiments are done using cells rather than tissues. Much of what we know about cancer, heritable diseases, and the effects of the environment on human health has been derived from studies of cultured cells.
What types of cells do people study, and from where do they come? Cells acquired directly from animal tissue are called primary cells. They are difficult to culture, largely because primary cells in this context divide only a limited number of times. This limitation on the lifespan of cultured primary cells, called the Hayflick limit, is a problem because it requires a researcher to repeatedly remove tissues from animals to complete a study. Cell isolation processes can be quite labor-intensive, and also can complicate data analysis due to inherent animal-to-animal variation. To get around this problem, researchers study cells that are immortal, which means the cells are able to divide indefinitely. Though using immortal cells is preferable for many reasons, some inherent cell-to-cell variation still exists in such populations and the genetic changes that cause immortality may affect experimental outcomes.
The art of tissue culture lies in the ability to create conditions that are similar to what a cell would experience in an animal, namely 37°C and neutral pH. Blood nourishes the cells in an animal, and blood components are therfore used to feed cells in culture. Serum, the cell-free (and clotting-factor free) component of blood, contains many of the factors necessary to support the growth of cells outside the animal. Consequently, serum is frequently added to tissue culture medium, although serum-free media (also called chemically defined media) exist and support some types of cultured cells.
Cultured mammalian cells must grow in a germ-free environment and researchers using tissue culture must be skilled in sterile or asceptic technique. Bacteria double very quickly relative to mammalian cells. An average mammalian cell doubles about once per day whereas many common bacteria can double every 20 minutes under optimal conditions. Consequently, if you were to co-culture 100 mammalian cells and 1 bacteria together in a flask, within 24 hours you would have ~200 unhappy mammalian cells, and about 100 million happy bacteria! Needless to say, you would not find it very useful to continue to study the behavior of your mammalian cells under these conditions.
Today you will learn and practice asceptic technique to collect and count cells that were previously cultured by the teaching faculty. You will use the cells you collect to seed (or culture) cells for a survival assay that will assess the affect of drug treatment following DNA damage. To ensure you are well-prepared for your work in the tissue culture room, your instructor will provide a demonstration before you begin. Because the tissue culture room is not large enough to accommodate the entire class at once, we will be split into two groups today...and all subsequent days that involve tissue culture. Half of the class will begin in tissue culture with Part 1 and the other half of the class will begin by preparing previously seeded DLD-1 and BRCA2- cells for Western blot analysis. Your instructor will designate the groups.
Protocols
Part 1: Practice tissue culture techniques
Part 1a: Demonstration of best practices
Part 1b: Seed cells for survival assay
Part 2: Prepare cell lysates for Western blot
Reagents
Next day: Complete Western blot and induce DNA damage for survival and quantitative PCR assays
Previous day: Last day of M1