Difference between revisions of "Spring 2012:Leanna Morinishi Lab 2"
From Course Wiki
(→Results) |
|||
| Line 42: | Line 42: | ||
== Results == | == Results == | ||
| − | [[File:PSF-3Dview2012.png| | + | {| width="80%" align="center" |
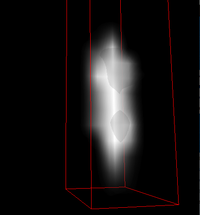
| − | [[File:PSF32012.gif | + | | [[File:PSF-3Dview2012.png|200px|thumb|<b>Figure 1.</b> A 3D view of our PSF found by looking at the light recorded by the microscope by a single fluorescent 190 nm bead.]] |
| + | | [[File:PSF32012.gif|thumb|<b>Figure 2.</b> A image stack of our PSF found by looking at a 190 nm fluorescent bead.]] | ||
| + | |} | ||
<br style="clear: both" /> | <br style="clear: both" /> | ||
Revision as of 02:37, 20 March 2012
Contents
Lab 2: Fluorescence Microscopy
Goals
In this module, I am interested in:
- Building and optimizing a fluorescent microscope
- Learning to control the microstepper
- Learning to talk to and control the camera
- Learning more about 3d deconvolution
- Test how it deconvolution is improved with actual PSF vs. theoretical PSF of the microscope
Methodology
Materials
- Pololu A4983 Stepper Motor Driver Carrier with Voltage Regulators[1]
- Thorlabs 8 mm Travel Stepper Motor Actuator[2]
- Matlab
- Matlab Image Acquisition Toolbox[3]
- Matlab Allied Vision Technologies Adaptor[4]
- National Instruments USB Data Acquisition[5]
- Epi-fluorescence Microscope[6] with parts from Thorlabs
- Submicron beads, small enough to be considered point sources
Deconvolution Methods for 3D Fluorescence Microscopy Images[7]
Microscope construction
- The microscope was a reanimation of the teaching microscope from the 20.309 lab. We changed the lenses a bit.
- The scope contains a 8.33x beam expander and 40x objective as of now. The objectives are changeable, but require some adjustment of the 200mm tube lens.
Laser Alignment
- The neutral density filter ensures we do not burn our eyeballs while aligning the laser. 532 nm protective goggles should be worn when appropriate.
- T-shirt targets were used as guides to center the expanded beam with the mirrors.
- With barrier filter still in place, we used a fluorescent reference slide and Steve's Super Special Software (or something like that) called Capture Image or something to look at the intensity histogram and redirect the mirror just prior to the CCD camera to maximize light homogeneity.
- Profit.
PSF Scanning
- Open GUI
- Turn on sleep, manually focus on psf sub-micron fluorescent beads (190 nm)
- Run psf scan on GUI
- Take image stack, open imageJ with deconvolution plugin
Results
Code
PSFmicrometer.m
- Establishes digital channel in 'Dev1'
- Adds 5 digital output lines
- In the future, user can set the zSampleDistance (aka the zdepth of the scan) and nyquistStepDistance
- Takes these values and determines which step size to use in microns and the value to convert to binary to send to the Driver in M1 M2 and M3
- Actual step size will always be the step smaller than the nyquistStepDistance
- Determines numberOfSteps which is just zdistance/stepdistance
- Moves Stage
- Half the zdistance back, quickly
- Full zdistance scan forward through the area of interest, slowly taking photos at each stop
- Half the zdistance back to original place
- Stops the channel
References
- ↑ A4983 Stepper Motor Driver Carrier with Voltage Regulators
- ↑ 8 mm Travel Stepper Motor Actuator
- ↑ Mathworks Image Acquisition Toolbox
- ↑ Matlab Allied Vision Technologies Adaptor
- ↑ National Instruments USB Data Acquisition 6210
- ↑ Lab_Manual:Optical_Microscopy
- ↑ Deconvolution Methods for 3D Fluorescence Microscopy Images - WUSTL 2006