Difference between revisions of "Optical Microscopy Part 2: Fluorescence Microscopy"
(→Overview) |
|||
| Line 5: | Line 5: | ||
==Overview== | ==Overview== | ||
| − | In | + | In this part of the microscopy lab, you will add epi-fluorescence imaging capability to your microscope. You will use a 5 mW, green (λ=532 nm) laser to illuminate fluorescent samples. When illuminated by the laser, the fluorescent samples will emit light in the orange-red region of the visible spectrum (550-610 nm). You will image fluorescent microspheres of various sizes. Using an image of a fluorescence reference slide and a dark image, you will correct the images to compensate for nonuniform illumination. |
| − | {{Template:Safety Warning|message=Do not begin working with the laser until you are familiar with laser safety procedures. If you missed laser safety training, see an instructor.}} | + | ===Laser safety=== |
| + | {{Template:Safety Warning|message=You will use a 5mW, 532 nm laser with focusing optics in this lab. Do not begin working with the laser until you are familiar with laser safety procedures. If you missed laser safety training, see an instructor.}} | ||
While you work on this part of the lab, keep these laser safety best practices in mind: | While you work on this part of the lab, keep these laser safety best practices in mind: | ||
| Line 39: | Line 40: | ||
====Dichroic mirror and barrier filter==== | ====Dichroic mirror and barrier filter==== | ||
| − | |||
| − | |||
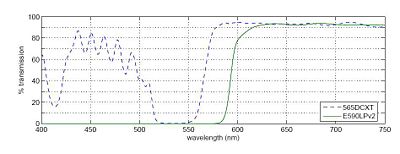
In any fluorescence system, a key concern is viewing only the emitted fluorescence photons, and eliminating any background light, especially from the illumination source. Two optical elements address the problem. A dichroic mirror reflects light of one wavelength, and passes light of another. The transmission spectrum for the 565DCXT from Chroma Technologies, which is similar to your NT47-268 dichroic from Edmund Optics, is shown in figure below. In contrast, a barrier filter blocks a particular spectral region extremely well. We will use the E590LPv2 barrier filter from Chroma Technologies. | In any fluorescence system, a key concern is viewing only the emitted fluorescence photons, and eliminating any background light, especially from the illumination source. Two optical elements address the problem. A dichroic mirror reflects light of one wavelength, and passes light of another. The transmission spectrum for the 565DCXT from Chroma Technologies, which is similar to your NT47-268 dichroic from Edmund Optics, is shown in figure below. In contrast, a barrier filter blocks a particular spectral region extremely well. We will use the E590LPv2 barrier filter from Chroma Technologies. | ||
Revision as of 13:08, 19 September 2013
Overview
In this part of the microscopy lab, you will add epi-fluorescence imaging capability to your microscope. You will use a 5 mW, green (λ=532 nm) laser to illuminate fluorescent samples. When illuminated by the laser, the fluorescent samples will emit light in the orange-red region of the visible spectrum (550-610 nm). You will image fluorescent microspheres of various sizes. Using an image of a fluorescence reference slide and a dark image, you will correct the images to compensate for nonuniform illumination.
Laser safety
While you work on this part of the lab, keep these laser safety best practices in mind:
- Use the correct laser safety eyewear. Always check the marking on the goggles.
- Know the beam path at all times.
- Your microscope directs the laser light upward.
- Do not allow the unattenuated laser beam to shine on the ceiling. Stop the beam if you are not using an ND filter.
- Never put your face directly above the objective.
- Use an OD = 2 neutral density filter to reduce the laser power to a safe level when making adjustments.
- Remove jewelry and reflective clothing items.
- Confine the beam inside lens tubes.
- Use a stop to prevent uncontained beams.
- Turn of the laser when you are using reflective tools.
- Never point the laser toward other people.
- Disable lasers when they are not in use.
Background reading
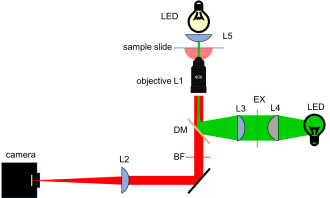
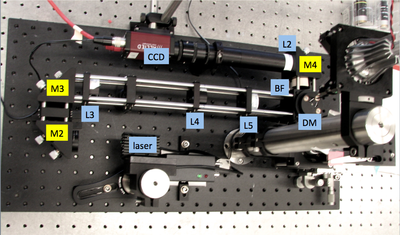
Microscope block diagram and implementation=
|
20.309 microscope block diagram |
20.309 student's microscope |
|---|
Dichroic mirror and barrier filter
In any fluorescence system, a key concern is viewing only the emitted fluorescence photons, and eliminating any background light, especially from the illumination source. Two optical elements address the problem. A dichroic mirror reflects light of one wavelength, and passes light of another. The transmission spectrum for the 565DCXT from Chroma Technologies, which is similar to your NT47-268 dichroic from Edmund Optics, is shown in figure below. In contrast, a barrier filter blocks a particular spectral region extremely well. We will use the E590LPv2 barrier filter from Chroma Technologies.
The figure below shows the transmittance of the dichroic mirror and barrier filter over a range of wavelengths. The barrier filter is essential for high-sensitivity fluorescence imaging as it will block any reflected green light while allowing trace amount of red light from the fluorophores. It will also pass the red light from the LED in the illuminator for bright field imaging so that you can accomplish combined bright-field and fluorescent imaging to visualize your sample.
Samples
You have the following samples available for imaging with both 40× and 100× objectives:
- A red fluorescence reference slide
- Several types of sample slides with 3.26 μm or 0.84 μm red-fluorescent beads (several different dyes, but with peak excitation roughly 535nm, peak emission roughly 600nm)
- PSF slides with 110 nm or 170 nm red-fluorescent beads
Lab Procedures
Add laser illumination path to your microscope
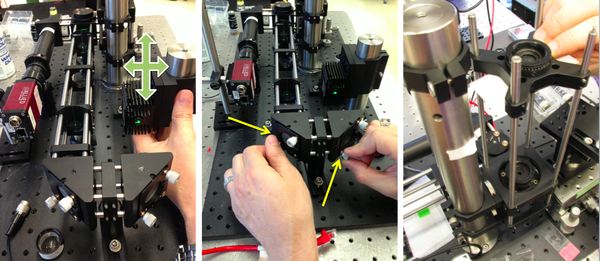
- Go over your design with one of the instructors before you start building.
- Use cage rods to construct a cage of appropriate size for the beam expander and excitation tube lens (L3, L4, and L5).
- For maximum flexibility in positioning, removing, and reinstalling optics, use 3 cage rods instead of 4. Use a cage plate (CP02) for each lens. Mount the lens in a 1/2" lens tube (SM1L05) so it can be easily removed or installed.
- Add the two turning mirrors to the end of the cage.
- Use a thick cage plate (CP02T) only to join 2 cages. Don't use CP02T to mount lenses — there are not enough of them in the lab to use this way. It is easy to tell the difference. CP02T has 8 set screws and is roughly twice as thick as the CP02; CP02 has 4 set screws.
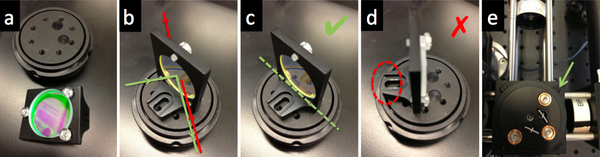
- Use a kinematic plate (B4C) to mount dichroic mirror DM on a cube optic mount (B5C).
- The first surface of the dichroic should face the laser.
- Some dichroics have an arrow indicating the first surface. If not, to ascertain which surface has the coating, watch the reflection of a corner of a piece of lens paper as it touches the mirror. On the first surface, the corner and its reflection will appear to touch. Held the other way, the corner and its image will appear a few millimeters apart.
- Mount the dichroic so that the first surface lies on exactly a diameter of the B5C mount.
- The mounting bracket should not stand in the way of the rotation of the kinematic stage.
- Use the clear plastic #4/40 screws affix the dichroic mirror without deforming nor scratching its surface.
- Hold the screwdriver at the tip to avoid slipping and scratching the dichroic. The screwdriver will instantly scratch the dichroic.
- Ask an instructor for help if you need to clean a dichroic mirror or barrier filter. Dichroics have delicate, exposed coatings and must be cleaned with extra care.
- The first surface of the dichroic should face the laser.
- Use four black, plastic #4/40 screws to hold the cube optic mount (B5C) on the cage cube (C6W).
- Tighten the screws enough so that the cube optic mount holds its adjustment, but can still be rotated.
Align the laser illumination path
- Remove L3, L4, L5, and the objective lens L1.
- Mount the laser.
- Insert a neutral density filter (ND filter) between the laser and M2 to reduce the laser power tow a safe level for adjustment.
- Remove jewelry. Turn of the laser before using reflective tools.
- Turn on the laser. Use a beam stop until the laser position is set. Adjust the laser position so that the laser shines near the middle of M2.
- Adjust M2 and M3 to center the laser light in the cage.
- Use two CPA1 alignment targets to gauge beam alignment.
- Adjust the dichroic mirror DM so that the beam will enter the middle of the objective lens.
- Use a DG10 mounted frosted-glass alignment disk with a pinhole to show the optical center of the vertical beam path.
- Verify that the centered beam is perpendicular to the floor. If the beam is at an angle, verify that the dichroic mirror DM is mounted on a diameter of the rotating mount.
- Replace the beam expander lenses, L3 and L4. Adjust the separation between L3 and L4 to achieve a collimated beam.
- It may be necessary to make small adjustments to M2 and M3 to recenter the beam.
- Replace the excitation tube lens L5 and the objective lens L1. Adjust the position of L5 for best beam collimation.
- The product of beam divergence and diameter is constant. L5 and L1 shrink the beam, causing increased divergence. The beam emerging from the objective will likely appear to grow in size as it propagates, even when the lenses are in their optimal positions.
- It may be necessary to make small adjustments to M2 and M3 to recenter the beam.
Barrier filter
To easily switch back and forth between bright-field and fluorescence microscopy mode, you may need to remove and add the barrier filter (BF) from your setup (this is obviously true and mandatory if your bright-field light source is a blue LED, whose transmitted light would otherwise be blocked by the E590LPv2 BF; the latter only transmits light of wavelength > 590 nm).
- Keeping in mind that barrier filters are designed to work under collimated light illumination, where do you intend to position the BF in your microscope light path?
- What wavelengths must your BF transmit?
- The barrier filter removes any light from the illumination laser that was not reflected by the dichroic mirror.
- Ask an instructor for help if you need to clean a dichroic mirror or barrier filter. (These optics have exposed coatings and must be cleaned with extra care.)
- During fluorescent imaging, you will not use the bright field illuminator. Bright-field capability is useful for first visualizing the sample and viewing what features are in the field of view. Your design should retain the ability to do both white light and fluorescent visualization.
Fluorescence Imaging
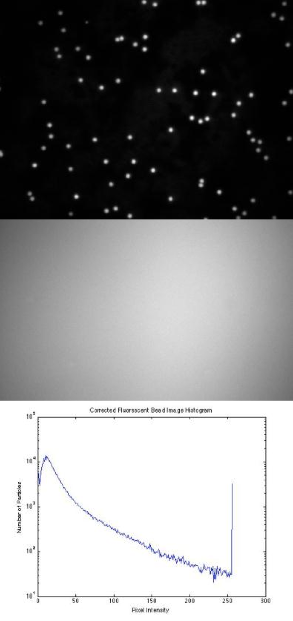
- Use a fluorescence reference slide to center the field of view and to optimize the uniformity of illumination. Take an image of this, with both the 40× and 100× objectives. Use the histogram display of the camera software (function imhist in Matlab) to be certain that the image is not overexposed and that you are using the full dynamic range of the camera.
- Under each magnification configuration (40× or 100×), take an image of the 3 μm and 1 μm red-fluorescent beads (their exact diameters are actually 3.26 μm and 0.84 μm). Perform flat-field correction on the image (i.e. divide the image by a normalized copy of your reference image). Be sure that your microscope is in the same state when you record the reference image and the bead image. [Note: Actively enable the 12-bit option of the camera software, which otherwise defaults to 8-bit imaging]. Compare what you see before and after flat field correction.
- Make initial images of the PSF slide. This slide consists of 110nm fluorescent beads, which are proxies for ideal point sources. The image of a practical point source object will closely approximate the ideal Airy disk. Calculate the approximate resolution of your microscope, using this measured Airy disk or Gaussian image (depending on the quality of your microscope and the resolution of the camera).
Measuring resolution
One of the most commonly used definitions of resolution is the distance between two point sources in the sample plane such that the peak of one source’s image falls on the first minimum of the other source’s image. This suggests a procedure for measuring resolution: image a point source; measure the peak-to-trough distance; and divide by the magnification. In this part of the lab, you will use this method to estimate the resolution of your microscope.
A practical problem with this method is that true point sources are difficult to come by. If you are using a telescope, stars are readily available approximate point sources. In microscopy, people usually use tiny, fluorescent beads with diameters of 100-190 nm. These beads are small enough to be considered point sources. You will use nonlinear regression to estimate the resolution of your microscope from an image of the tiny beads. Unfortunately, beads small enough for this purpose are not very bright. Imaging them can be challenging. Your microscope must be aligned very well to get good results.
You will use image processing functions to locate the beads in your image and fit a Gaussian function to them. Gaussians are more amenable to nonlinear regression than Bessel functions, and they are a very good approximation. It is straightforward to convert the Gaussian parameters to Rayleigh resolution. (See Converting Gaussian fit to Rayleigh resolution.)
- Make an image of a sample of 100 or 190 nm fluorescent beads with the 40X objective.
- Use 12-bit mode on the camera and make sure to save the image in a format that preserves all 12 bits.
- Use
imhistto ensure that the image is exposed properly.- Since there are a very small number of bright pixels, plot the histogram counts on a logarithmic scale.
- Include the image in your lab report.
- Use image processing functions to locate non-overlapping, single beads in the image.
- Use nonlinear regression to fit a Gaussian to each bead image.
- Convert the Gaussian parameters to resolution.
- Report the results in your lab report.
- Discuss how does the measured resolution compares with the theoretical value.
Example images:
Report: Microscope construction and fluorescence characterization
Microscope design
- Update the block diagram description of your microscope, its photograph, and annotation of pertinent optical components and distances.
Fluorescence imaging characterization
Demonstrate the epi-fluorescence imaging capability of your enhanced microscope:
- Provide the fluorescent reference image you collected with both the 40× and 100× objectives, as either an image (see imshow command in Matlab) or a surface plot (see surf command in Matlab), as well as a cross-section of signal intensity across its diagonal (see improfile command in Matlab).
- Exhibit your images of the 3 μm and 1 μm fluorescent bead samples, with both the 40× and 100× objectives. Compare and contrast these pictures before and after flat-field correction to counteract nonuniform illumination.
- Describe your flat-field correction procedure, from recording the reference image through applying the correction.
- Also provide log(y) scaled histograms of at least one original and corrected image pair.
Discussion on design and image quality
- Comment on your corrections and relate your results to your choices during beam expander design and construction.
- How do these choices relate to your intended experiments in weeks three and beyond?
Measured microscope resolution with 40× objective
- Provide a sample of the images used for resolution estimation (overlay the fit – see
plotgaussfitcommand). - Provide a table with measured estimates of FWHM resolution by Gaussian fitting for the 40× objective.
- Provide a bullet point outline of data analysis methodology.
- Comment on estimated versus theoretical value.
Optical microscopy lab
Code examples and simulations
- Converting Gaussian fit to Rayleigh resolution
- MATLAB: Estimating resolution from a PSF slide image
- Matlab: Scalebars
- Calculating MSD and Diffusion Coefficients