20.109(S23):M2D7
Contents
Introduction
In this laboratory session, you will complete the final two experiments of this module: analyzing metal uptake and tolerance in W303α cells expressing the Fet4_mutant you designed and generated.
In the last laboratory session, you prepared samples for analysis using inductively coupled plasma optical emission spectroscopy (ICP-OES). This technique is also known as ICP-AES, with the "A" representing "atomic". With this approach, a solution containing unknown elements is dissolved in acid to create a solution of soluble elements. These elements are pumped through a nebulizer to create a consistent fine spray. Inside the chamber, a quartz torch contained in an argon-cooled induction coil is lit, leading to the generation of a stable plasma "flame" capable of reaching 10,000K (the reported temperature on the surface of the sun).
As elements are aerosolized through the nebulizer, they are sprayed into the plasma where they are ionized with the electrons taking up the thermic energy emitted by the plasma and thus reaching an excited state. As the electrons fall back to ground level, they emit light at specific optical wavelengths that can be detected by the spectrometer. This approach allows us to determine the presence of elements in a sample by taking advantage of characteristic emission patterns of different elements.
To examine the ability of your transformed yeast to tolerate metal uptake, you will use a commercial assay to measure cell viability. The BacTiter-Glo Microbial Cell Viability Assay is a method for quantifying the number of viable cells based on measuring the amount of ATP present. ATP is a proxy for the presence of metabolically active (alive) cells. In this assay, the cells are lysed and ATP is released from the active cells. In a reaction catalyzed by a propriety luciferase enzyme, luciferin, ATP, and oxygen result in oxyluciferin, AMP, PPi, carbon dioxide, and light. The light product is then measured using a luminometer.
Protocols
Part 1: Perform metal tolerance experiment
- Obtain your Fet4_mutant culture from the front bench.
- Untransformed W303α and W303α transformed with Fet4 will be tested in parallel by the teaching faculty.
- Gently tritruate with a 1ml pipette to create a homogeneous suspension.
- Obtain cuvettes from the front bench to measure the OD600 of each culture prior to beginning the experiment.
- Add 1ml of each cell suspension to individual cuvettes and read on the spectrophotometer.
- Use 1ml SD-G for a blank.
- Record these numbers in your notebook.
- Using SD-G media as a diluent, dilute each of your cultures to OD600 ~ 1.0 and a final volume of 8ml. This does not have to be exact, but the cultures should have a similar OD600 before you begin the experiment.
- Add CdCl2 for a final concentration of 100μM in each culture.
- Incubate your labeled tubes shaking at 30°C for 2.5 hours to allow uptake.
- During this incubation time, complete Part 2 and 3 of the wiki.
- Following incubation, take an additional OD600 reading to account for any changes in culture density, and allow normalization of data across groups.
- Record this in your notebook.
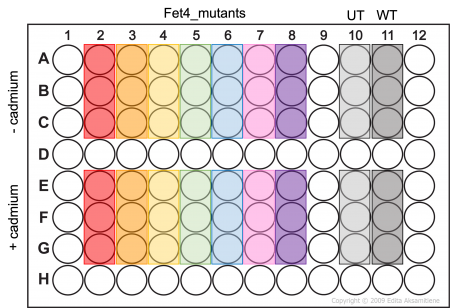
- Add 100μL of cell suspension to the appropriate wells of an opaque-walled 96 well plate as shown in the plate map (Fet4_mutant cells -/+ cadmium will be assayed in triplicate).
- Add 100μL BacTiter-Glo reagent to each well containing cell suspension and mix.
- Incubate plate on orbital shaker, protected from light, for 5 minutes.
- Read the plate on a luminometer plate reader.
Part 2: Review collection of ICP-OES data
Due to the training required to operate the machinery, the teaching faculty have generated the ICP-OES data you will analyze. Please review the following steps to help conceptualize how the data were generated.- Enter the ICP-OES facility and place samples in fume hood while setting up the equipment.
- Open the liquid argon regulator valve to begin the flow of argon gas to the ICP apparatus.
- Turn on the chiller to cool the ICP apparatus during operation.
- Tighten tubing connecting autosampler to nebulizer.
- Open the ICP Expert program and temporarily boost the argon gas flow to purge the system and maintain steady gas flow.
- Use the instrumentation panel in ICP Expert to light the plasma torch.
- Plasma must be burn for 20 minutes before data can be collected.
- Ensure collection probe is place in rinse solution.
- Place samples in autosampler.
- Before beginning experimental run, verify the following conditions to ensure machine is operational.
- Periodic bubbles present in drain lines for spray chamber and autosampler.
- Stable fog present in nebulizer sample chamber.
- Stable torch flame.
- Set the number of standards and samples on the autosampler map to set order of sample processing.
- Set parameters of sampling as listed below:
- 3 replicates per sample
- RF power = 1.2 kW
- pump speed = 12rpm
- read time = 5s
- rinse time = 30s
- stabilization time = 15s
- nebulizer flow = 0.70 L/min
- plasma flow = 12 L/min
- radial viewing mode
- viewing height = 8mm
- Set element and wavelength to be tested
- If unsure of which wavelength is ideal for elements under analysis, select all available wavelengths
- Save program file.
- Begin ICP-OES testing program.
Part 3: Analyze ICP-OES data
- Using this interactive period table here, specifically designed for IPC analysis, consider the following questions and note them in your laboratory notebook.
In your laboratory notebook, complete the following:
- What
Reagents list
- BacTiter-Glo Microbial Cell Viability Assay solution (Promega)
- Synthetic dropout - galactose (SD-G) media: 0.17% yeast nitrogen base without amino acid and ammonium sulfate (BD Bacto), 0.5% ammonium sulfate (Sigma), 0.192 % amino acid mix lacking uracil (Sigma), 2% raffinose (Sigma), 2% galactose (Sigma), 0.1% adenine hemisulfate (Sigma)
- Cadmium chloride (Sigma), stock concentration= 100mM
- TraceCERT cadmium standard for ICP (Sigma)
- TraceCERT iron standard for ICP (Sigma)
Next day: Complete data analysis and organize Research article figures