20.109(F20):M3D2
Contents
Introduction
In the previous laboratory session, we researched how to best target a gene using the CRISPRi system. The goal for today is to review the strategy used in constructing an expression plasmid that encodes the sgRNA_target. This will allow for transcription of the sgRNA_target molecule and ultimately provides the specificity needed to target dCas9 to a gene of interest. The strategy used in constructing the psgRNA_target plasmid involved two common methods: primer design and polymerase chain reaction.
Primer design
To amplify a specific sequence of DNA, you first need to design primers -- one primer that anneals at the start of the sequence of interest (the 5' end) and a second primer that anneals at the end of the sequence of interest (the 3' end). The primer that anneals at the start of the sequence is referred to as the 'forward' primer. The forward primer anneals to the non-coding DNA strand and reads toward, or into, the gene of interest. The 'reverse' primer anneals to the coding DNA strand at the end of the sequence and reads back into the sequence. Primers can also be useful in adding sequence to sequences upon amplification via the polymerase chain reaction.
Polymerase chain reaction (PCR)
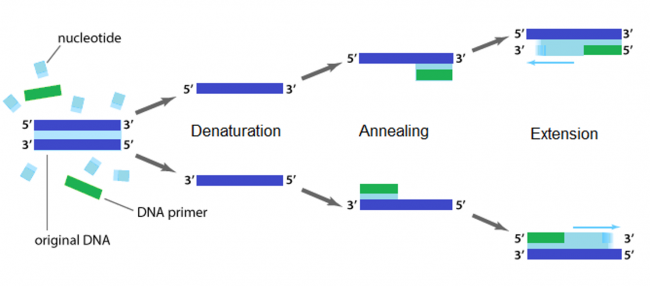
The applications of PCR are widespread, from forensics to molecular biology to evolution, but the goal of any PCR is the same: to generate many copies of DNA from a single or a few specific sequence(s) (called the “template”). In addition to the template, PCR requires only three components: primers to bind sequence flanking the target, dNTPs to polymerize, and a heat-stable polymerase to catalyze the synthesis reaction over and over and over. DNA polymerases require short initiating pieces of DNA called primers to copy DNA. In PCR amplification, forward and reverse primers that target the non-coding and coding strands of DNA, respectively, are separated by a distance equal to the length of the DNA to be copied. To amplify DNA, the original DNA segment, or template DNA, is denatured using heat. This separates the strands and allows the primers to anneal to the template. Then polymerase extends from the primer to copy the template DNA. How many cycles of PCR are required to achieve the desired double-stranded amplification product?
Several features are important to consider when designing primers for PCR. Primers that are too short may lack requisite specificity for the desired sequence, and thus amplify an unrelated sequence. The longer a primer is, the more favorable are its energetics for annealing to the template DNA, due to increased hydrogen bonding. On the other hand, longer primers are more likely to form secondary structures such as hairpins, leading to inefficient template priming. Two other important features are G/C content and placement. Having a G or C base at the end of each primer increases priming efficiency, due to the greater energy of a GC pair compared to an AT pair. The latter decrease the stability of the primer-template complex. Overall G/C content should ideally be 50 +/- 10%, because long stretches of G/C or A/T bases are both difficult to copy. The G/C content also affects the melting temperature. PCR is a three-step process (denature, anneal, extend) and these steps are repeated 20 or more times. After 30 cycles of PCR, there could be as many as a billion copies of the original template sequence.
Protocols
Part 1: Practice sgRNA design principles
To ensure that you understand the principles of using sgRNA to target a gene of interest, complete the sgRNA Design Worksheet with your laboratory partner (linked here).
In your laboratory notebook, attach the completed sgRNA Design Worksheet.
After identifying the sgRNA_target sequence that is best for targeting the gene of interest in the host genome, the sgRNA_target is inserted into an expression plasmid. The expression plasmid used for regulating gene expression via the CRISPRi system is described in the following article:
Larson et al. "CRISPR interference (CRISPRi) for sequence-specific control of gene expression." Nature. (2013) 8:2180-2196.
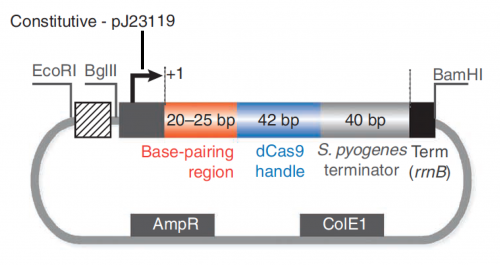
In this exercise, you will explore the features present in the plasmid that are necessary to express the sgRNA_target sequence (see plasmid map below).
In your laboratory notebook, complete the following:- Review the details provided in the caption for Fig. 1B of the Larson et. al. article.
- Describe the purpose / role for each of the following features that are present in the bacterial sgRNA plasmid. Please note: you many need to reference resources outside of the article!
- Constitutive - pJ23119
- Base-pairing region
- dCas9 handle
- S. pyogenes terminator
- Term (rrnB)
- AmpR
- ColE1
- +1
- restriction enzyme recognition sequences: EcoRI, BglII, and BamHI
Part 2: Review sgRNA expression plasmid construction strategy
Synthesize sgRNA_target sequence
INCLUDE THAT CAS9 HANDLE SEQUENCE ADDED (WITH VISUAL?)
Before we continue, we should consider how the sgRNA_target sequences that were identified via examining DNA sequences were used to generate actual primers that can be used to amplify DNA. Current oligonucleotide (primer) synthesis uses phosphoramidite monomers, which are simply nucleotides with protection groups added. The protection groups prevent side reactions and promote the formation of the correct DNA product. The DNA product synthesis starts with the 3'-most nucleotide and cycles through four steps: deprotection, coupling, capping, and stabilization. First, deprotection removes the protection groups. Second, during coupling the 5' to 3' linkage is generated with the incoming nucleotide. Next, a capping reaction is completed to prevent uncoupled nucleotides from forming unwanted byproducts. Lastly, stabilization is achieved through an oxidation reaction that makes the phosphate group pentavalent. For a more detailed description of this process, read this article from IDT DNA.
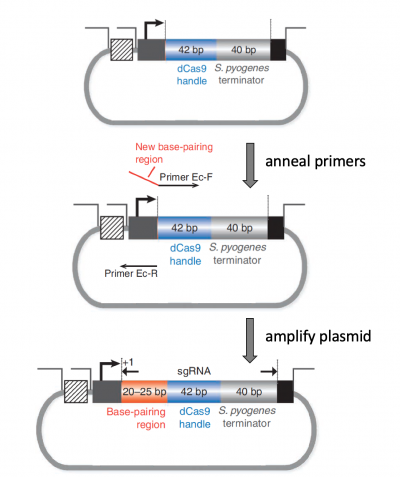
Insert sgRNA_target sequence into expression plasmid
With a primer synthesized according to the process described above, the Q5 Site Directed Mutagenesis Kit from NEB was used to insert the sgRNA sequence into the expression plasmid. For this, a reaction was prepared with the following: the sgRNA_target primer, a universal CRISPRi primer specific to the expression plasmid, and the expression plasmid.
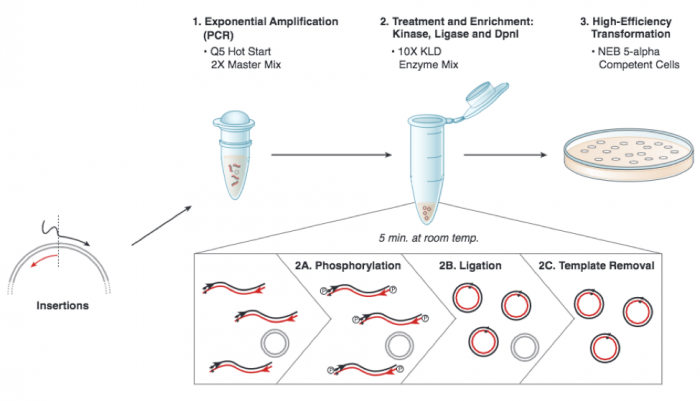
A more detailed depiction of the protocol used to insert an sgRNA_target sequence into the expression plasmid is included below. DNA polymerase will copy the plasmid using the gRNA primer to insert the target sequence you selected. Following this reaction the 'mutated' product is a linear DNA fragment. To generate circular plasmids that carry the gRNA sequence, the DNA is phosphorylated then ligated. In addition, there is still parental -- that is, non-mutant -- DNA present in your reaction product. To ensure that only the gRNA-containing plasmid is used in the next steps, the parental DNA is selectively digested using the DpnI enzyme. The underlying selective property is that DpnI only digests methylated DNA. Therefore, the synthetically made (and thus non-methylated) mutant DNA is not digested, while the parental DNA is digested due to methylation by the host bacterial strain originally used to amplify it. The resulting small linear pieces of parental DNA are simply degraded by the bacteria upon transformation, whereas the intact (due to the phosphorylation and ligation reaction) circular mutant DNA is amplified by the bacteria.
Each group will set up one reaction, for your insertion. Meanwhile, the teaching faculty will set up a single positive control reaction, to ensure that all the reagents are working properly. You should work quickly but carefully, and keep your tube in a chilled container at all times. Please return shared reagents to the ice bucket(s) from which you took them as soon as you are done with each one.
- Get a PCR tube and label the top with your team color and lab section (write small!).
- Add 10.25 μL of nuclease-free water.
- Add 1.25 μL of your primer mix (each primer should be at a concentration of 10 μM).
- Add 1 μL of pgRNA plasmid DNA (concentration of 25 ng/μL).
- Lastly, use a filter tip to add 12.5 μL of Q5 Hot Start High-Fidelity 2X Master Mix - containing buffer, dNTPs, and polymerase - to your tube.
- Once all groups are ready, we will begin the thermocycler, under the following conditions:
| Segment | Cycles | Temperature | Time |
|---|---|---|---|
| Initial denaturation | 1 | 98 °C | 30 s |
| Amplification | 25 | 98 °C | 10 s |
| 55 °C | 30 s | ||
| 72 °C | 2 min | ||
| Final extension | 1 | 72 °C | 2 min |
| Hold | 1 | 4 °C | indefinite |
- After the cycling is completed, you will complete the KLD reaction (which stands for "kinase, ligase, DpnI") using
- 1 μL of your amplification product
- 5 μL 2X KLD Reaction Buffer
- 1 μL KLD Enzyme Mix, and
- 3 μL nuclease-free water.
- Incubate the reaction for 5 min at room temperature.
Propagate psgRNA_target expression plasmid in competent cells
- Then, use 5 μL of the KLD reaction product to complete a transformation into an E. coli strain (NEB 5α cells of genotype fhuA2 Δ(argF-lacZ)U169 phoA glnV44 Φ80 Δ(lacZ)M15 gyrA96 recA1 relA1 endA1 thi-1 hsdR17) that will amplify the plasmid such that you are able to confirm the appropriate insertion (or 'mutation') was incorporated. The transformation procedure will be as follows:
- Add 5 μL of KLD mix to 50 μL of chemically-competent NEB 5α.
- Incubate on ice for 30 min.
- Heat shock at 42 °C for 30 s.
- Incubate on ice for 5 min.
- Add 950 μL SOC and gently shake at 37 °C for 1 h.
- Spread 50 μL onto LB+Amp plate and incubate overnight at 37 °C.
Confirm psgRNA_target with sequencing
Part 3: Align sgRNA_target sequences to host genome
Reagents list
- Q5 Site Directed Mutagenesis Kit (from NEB)
- Q5 Hot Start High-Fidelity 2X Master Mix: propriety mix of Q5 Hot Start High-Fidelity DNA Polymerase, buffer, dNTPs, and Mg2+
- 2X KLD Reaction Buffer
- 10X KLD Enzyme Mix: proprietary mix of kinase, ligase, and DpnI enzymes
- Universal CRIPSRi reverse primer 5' - ACT AGT ATT ATA CCT AGG ACT GAG CTA GC - 3'
- SOC medium: 2% tryptone, 0.5% yeast extract, 10 mM NaCl, 2.5 mM KCl, 10 mM MgCl2, 10 mM MgSO4, and 20 mM glucose
- LB+Amp plates
- Luria-Bertani (LB) broth: 1% tryptone, 0.5% yeast extract, and 1% NaCl
- Plates prepared by adding 1.5% agar and 100 μg/mL ampicillin (Amp) to LB
Next day: Analyze ethanol yield data