Difference between revisions of "Assignment 2 Part 3: Build an epi-illuminator for your microscope"
Juliesutton (Talk | contribs) (→Overview) |
Juliesutton (Talk | contribs) (→Add illuminator, dichroic mirror, and barrier filter) |
||
| Line 48: | Line 48: | ||
}} | }} | ||
| − | ==Add illuminator | + | ==Add fluorescence capabilities to your microscope== |
| + | |||
| + | ===Add illuminator and dichroic mirror=== | ||
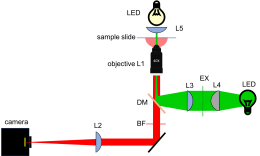
[[Image:LedEpifluorescenceMicroscope.png|thumb|center|400px|Layout of fluorescence microscope.]] | [[Image:LedEpifluorescenceMicroscope.png|thumb|center|400px|Layout of fluorescence microscope.]] | ||
| − | # Go over your illuminator design with one of the instructors before you start building. | + | Use your design from Part 2 and the example microscope to guide you in building the fluorescence part of your microscope. Here are some useful tips to keep in mind: |
| + | # Go over your illuminator design with one of the instructors before you start building if there are any parts you're unsure about. | ||
# For maximum flexibility in positioning, removing, and reinstalling optics, use 3 cage rods instead of 4. | # For maximum flexibility in positioning, removing, and reinstalling optics, use 3 cage rods instead of 4. | ||
| − | # You want the 75 mm excitation tube lens to be as close as possible to the objective. Use two retaining rings to secure the lens at the inlet of the C6W cage cube. | + | # You'll want the 75 mm excitation tube lens to be as close as possible to the objective. Use two retaining rings to secure the lens at the inlet of the C6W cage cube. |
#*[[Image:ExcitationTubeLensC6W.jpg|center|thumb|200px|Mount the excitation tube lens in the C6W cage cube.]] | #*[[Image:ExcitationTubeLensC6W.jpg|center|thumb|200px|Mount the excitation tube lens in the C6W cage cube.]] | ||
| − | # In the second half of the semester, you will upgrade your microscope to make two-color images. To avoid having to disassemble your microscope | + | # In the second half of the semester, you will upgrade your microscope (even more!) to make two-color fluorescence images. To avoid having to disassemble your microscope, you'll leave an extra cage cube (C6W) in between your green LED and the excitation tube lens. Mounting the cube to a post will also provide some stability and rigidity to your illuminator. |
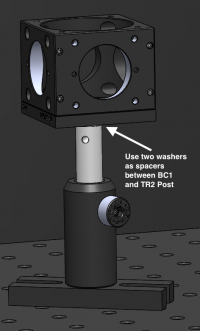
#* As before, the B1C cage cube cover plate has a shallow 8-32 threaded hole. Be sure to use 2 washers in between your TR2 post and the B1C base to be able to tighten the screw adequately without it getting stuck. | #* As before, the B1C cage cube cover plate has a shallow 8-32 threaded hole. Be sure to use 2 washers in between your TR2 post and the B1C base to be able to tighten the screw adequately without it getting stuck. | ||
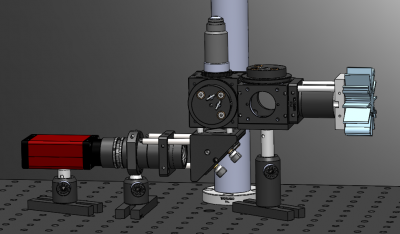
#* [[Image:FilterCubePostAssembly.png|center|thumb|200px|Mounting a second filter cube to a TR2 post and securing it in a PH2 post holder will provide the illuminator stability and allow you to expand your microscope for two colors later in the class.]] | #* [[Image:FilterCubePostAssembly.png|center|thumb|200px|Mounting a second filter cube to a TR2 post and securing it in a PH2 post holder will provide the illuminator stability and allow you to expand your microscope for two colors later in the class.]] | ||
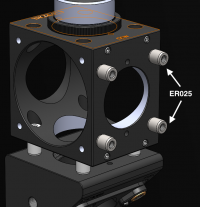
| − | #* Use the tiny 1/4" cage rods to connect the two C6W cages together | + | #* Use the tiny 1/4" cage rods (ER025) to connect the two C6W cages together. |
#*[[Image:FilterCubeConnection.png|center|thumb|200px|Use ER025 cage rods to connect two cage cubes.]] | #*[[Image:FilterCubeConnection.png|center|thumb|200px|Use ER025 cage rods to connect two cage cubes.]] | ||
| − | # Mount the aspheric lens in a 1/2" lens tube (SM1L05). Use two retaining rings like you did in Assignment 1 to mount your 25 mm lens for the trans illuminator. The | + | # Mount the aspheric lens in a 1/2" lens tube (SM1L05). Use two retaining rings like you did in Assignment 1 to mount your 25 mm lens for the trans illuminator. The 20 mm asphere looks very similar to the 25 mm plano convex lenses used previously, so make sure to label it appropriately. |
# Use some plastic pipe as a sleeve around the bright green LED to protect your eyes. | # Use some plastic pipe as a sleeve around the bright green LED to protect your eyes. | ||
#*[[Image:PlasticPipeSleeve.jpg|center|thumb|200px|Use plastic pipe to protect your eyes from the bright LEDs.]] | #*[[Image:PlasticPipeSleeve.jpg|center|thumb|200px|Use plastic pipe to protect your eyes from the bright LEDs.]] | ||
| Line 87: | Line 90: | ||
#* We don't know exactly where the BFP is, but it is roughly near the blue line on the 40x objective. | #* We don't know exactly where the BFP is, but it is roughly near the blue line on the 40x objective. | ||
#* Use a piece of lens paper or a kimwipe and hold it roughly where you anticipate the BFP to be. Slide the green LED along its cage rods until an image forms on your paper. (Don't worry if it's not exact; we'll do some fine tuning to find the exact location.) Make sure your excitation beam is still centered and vertical before proceeding. | #* Use a piece of lens paper or a kimwipe and hold it roughly where you anticipate the BFP to be. Slide the green LED along its cage rods until an image forms on your paper. (Don't worry if it's not exact; we'll do some fine tuning to find the exact location.) Make sure your excitation beam is still centered and vertical before proceeding. | ||
| − | #* Replace the objective and insert a barrier filter in the | + | #* Replace the objective and insert a barrier filter in the afocal part of the imaging path. |
#* The barrier filter has delicate, exposed metal coatings. Ask an instructor for help if you need to clean it. | #* The barrier filter has delicate, exposed metal coatings. Ask an instructor for help if you need to clean it. | ||
# Use a fluorescence reference slide to center the field of view and to optimize the uniformity of illumination. | # Use a fluorescence reference slide to center the field of view and to optimize the uniformity of illumination. | ||
#* Start up the useful image acquisition tool and set the exposure to an appropriate level. | #* Start up the useful image acquisition tool and set the exposure to an appropriate level. | ||
#* Center the camera's FOV in the objective's FOV. | #* Center the camera's FOV in the objective's FOV. | ||
| − | #* Make small adjustments to the LED position in order to maximize the reference slide intensity | + | #* Make small adjustments to the LED position in order to maximize the reference slide intensity. |
| − | + | ||
| − | + | ||
==Fluorescence imaging== | ==Fluorescence imaging== | ||
Revision as of 20:31, 11 September 2018
Overview
It's time to start building! In this part of the assignment, you're going to modify your microscope to add the fluorescence capabilities we talked about in Part 2. You'll then test out these new capabilities by recording images of fluorescent microspheres. Finally, you'll process those images using a flat-field correction to adjust for non-uniform illumination. Once you've completed this assignment, you should be ready for next week when you'll make beautiful images of stained actin filaments inside of mammalian cells!
Laser safety
Add fluorescence capabilities to your microscope
Add illuminator and dichroic mirror
Use your design from Part 2 and the example microscope to guide you in building the fluorescence part of your microscope. Here are some useful tips to keep in mind:
- Go over your illuminator design with one of the instructors before you start building if there are any parts you're unsure about.
- For maximum flexibility in positioning, removing, and reinstalling optics, use 3 cage rods instead of 4.
- You'll want the 75 mm excitation tube lens to be as close as possible to the objective. Use two retaining rings to secure the lens at the inlet of the C6W cage cube.
- In the second half of the semester, you will upgrade your microscope (even more!) to make two-color fluorescence images. To avoid having to disassemble your microscope, you'll leave an extra cage cube (C6W) in between your green LED and the excitation tube lens. Mounting the cube to a post will also provide some stability and rigidity to your illuminator.
- As before, the B1C cage cube cover plate has a shallow 8-32 threaded hole. Be sure to use 2 washers in between your TR2 post and the B1C base to be able to tighten the screw adequately without it getting stuck.
- Use the tiny 1/4" cage rods (ER025) to connect the two C6W cages together.
- Mount the aspheric lens in a 1/2" lens tube (SM1L05). Use two retaining rings like you did in Assignment 1 to mount your 25 mm lens for the trans illuminator. The 20 mm asphere looks very similar to the 25 mm plano convex lenses used previously, so make sure to label it appropriately.
- Use some plastic pipe as a sleeve around the bright green LED to protect your eyes.
- Use a cube optic mount (B5C) to mount dichroic mirror DM on a kinematic platform (B4C). Place the kinematic platform in the cage cube (C6W).
- The first surface of the dichroic should face the excitation source.
- Some dichroics have an arrow indicating the first surface. If not, to ascertain which surface has the coating, watch the reflection of a corner of a piece of lens paper as it touches the mirror. On the first surface, the corner and its reflection will appear to touch. Held the other way, the corner and its image will appear a few millimeters apart.
- Mount the dichroic so that the first surface lies on exactly a diameter of the B5C mount.
- The mounting bracket should not stand in the way of the rotation of the kinematic stage.
- Use the clear plastic #4/40 screws affix the dichroic mirror without deforming nor scratching its surface.
- Hold the screwdriver at the tip to avoid slipping and scratching the dichroic. The screwdriver will instantly scratch the dichroic.
- Ask an instructor for help if you need to clean a dichroic mirror or barrier filter. Dichroics have delicate, exposed coatings and must be cleaned with extra care.
- The first surface of the dichroic should face the excitation source.
- Use four black, plastic #4/40 screws to hold the cube optic mount (B5C) on the cage cube (C6W).
- Tighten the screws enough so that the cube optic mount holds its adjustment, but can still be rotated.
- Make sure to block the (small percentage of) excitation light that will be transmitted through the dichroic mirror with some black lab tape affixed to the B5C cube optic mount.
Align the illumination path
- Use the lowest possible LED current when aligning to reduce risk of light getting in your eyes. Make sure to use some plastic pipe as a sleeve around the LED to reduce stray light, and to cover the back of the C6W cube with electrical tape. Don't hesitate to ask an instructor for help during the alignment process.
- Remove jewelry. Turn of the LED before using reflective tools.
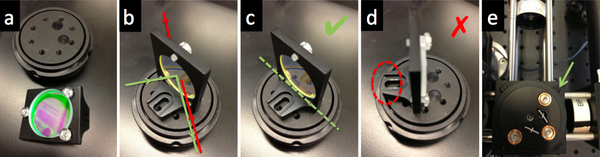
- Adjust the dichroic mirror DM so that the beam will enter the middle of the objective lens.
- Remove your objective and replace it with a DG10 mounted frosted-glass alignment disk with a pinhole to show the optical center of the vertical beam path.
- Connect your LED to the power supply. Adjust the current limit to 0.06A, and the voltage at 4V, and then enable the power. This should be very close to the minimum amount of light visible from your LED.
- Rotate the B4C platform until you see some light hitting your diffuser. Continue rotating until the pattern is centered. You will also likely need to use the adjustment screws to center the beam in both X and Y.
- Remove the DG10 frosted glass alignment tool. Use a piece of lens paper or a kimwipe to verify that the excitation light is both centered and directed perpendicular to the floor. If the beam is at an angle, verify that the dichroic mirror DM is mounted on a diameter of the rotating mount.
- Roughly align the LED condenser lens to make an image near the back focal plane of the objective
- We don't know exactly where the BFP is, but it is roughly near the blue line on the 40x objective.
- Use a piece of lens paper or a kimwipe and hold it roughly where you anticipate the BFP to be. Slide the green LED along its cage rods until an image forms on your paper. (Don't worry if it's not exact; we'll do some fine tuning to find the exact location.) Make sure your excitation beam is still centered and vertical before proceeding.
- Replace the objective and insert a barrier filter in the afocal part of the imaging path.
- The barrier filter has delicate, exposed metal coatings. Ask an instructor for help if you need to clean it.
- Use a fluorescence reference slide to center the field of view and to optimize the uniformity of illumination.
- Start up the useful image acquisition tool and set the exposure to an appropriate level.
- Center the camera's FOV in the objective's FOV.
- Make small adjustments to the LED position in order to maximize the reference slide intensity.
Fluorescence imaging
In this part of the lab, you will make images of fluorescent microspheres and correct them for nonuniform illumination. In order to do the correction, you will need a reference image and dark image in addition to the image of the sample.
- Read this page to learn about the flat-field correction.
- Reference Images: Take the reference image as close as possible in time to the sample images. Don't make any adjustments to your microscope between capturing the reference image and the sample image. For example, every time you move a mirror or re-align the laser, you will change the illumination profile and you must take a new reference. Adjusting the camera exposure and gain between recording the reference and sample images is okay.
- Dark images: Each time you record an image (reference or sample), make sure to take a corresponding dark image using the EXACT SAME camera settings (i.e. use the exact same Exposure Time and Gain settings you had chosen for your reference/sample image). This is the only valid way to subtract the correct dark value from your reference/sample image. Use 12-bit imaging mode to get the best results.
- Read through this page to refresh you memory on saving in MATLAB. We recommend using the save command to save data as a .mat file that you can load back into MATLAB later. This makes it easy to reanalyze your images if necessary. You could also save in an image file format with imwrite. Convert the image to 16-bit, unsigned integer format with the correct range before saving.
Take images
- Record a reference image
- Put in an ND filter and take an image of the reference slide with the 40X objective.
- Turn off the laser and record a dark image (without changing any camera settings!)
- Use MATLAB to generate a histogram of to be certain that the image is exposed correctly.
- Remove the ND filter and image the samples:
- 0.84 μm beads and a corresponding dark image
- 3.26 μm beads and a corresponding dark image
- For each of these samples remember to make sure the image is exposed correctly, and to take a corresponding dark image
- Adjust the gain (to zero) and exposure of the camera to get the best picture.
Flat field correction
- Perform flat-field correction on the images.
- Divide the image by a normalized version of your reference image minus the dark image (see this page for more detail).
- Tip: If all your images are appearing dark in MATLAB you probably have not rescaled your image properly. Read through the displaying images section of this page if you need a refresher.
- Overview
- Part 1: Noise in images
- Part 2: Fluorescence microscopy
- Part 3: Build an epi-illuminator for your microscope