Difference between revisions of "20.109(S24):M2D4"
Becky Meyer (Talk | contribs) (→Introduction) |
Becky Meyer (Talk | contribs) (→Introduction) |
||
| Line 3: | Line 3: | ||
==Introduction== | ==Introduction== | ||
| − | Today you will analyze data produced by Sanger sequencing. In the sequencing section of the wiki yesterday, you added primers to your purified plasmids in order to amplify the section of plasmid which hopefully holds your insert. At the sequencing facility, the DNA plasmid and primer mixture was incubated with polymerase and dNTPs (deoxynucleotides). As part of a PCR reaction, the primer will bind to the complementary site on the plasmid and extend the nascent chain with base pairs, utilizing the appropriate dNTP. The sequencing facility also includes a set of fluorescently labeled ddNTPs (dideoxynucleotides). When these are incorporated, they will terminate the nascent chain because no new base can be linked. The resulting DNA fragment will | + | Today you will analyze data produced by Sanger sequencing of your mini-preps. In the sequencing section of the wiki yesterday, you added primers to your purified plasmids in order to amplify the section of plasmid which hopefully holds your insert. At the sequencing facility, the DNA plasmid and primer mixture was incubated with polymerase and dNTPs (deoxynucleotides). As part of a PCR reaction, the primer will bind to the complementary site on the plasmid and extend the nascent chain with base pairs, utilizing the appropriate dNTP. The sequencing facility also includes a set of fluorescently labeled ddNTPs (dideoxynucleotides). When these are incorporated, they will terminate the nascent chain because no new base can be linked. The resulting DNA fragment will end with a fluorophore corresponding with the final ddNTP base added. As the ddNTP is incorporated randomly during the extension process, the resulting pool of DNA fragments are over different sizes. These fragments are sorted by size and passed by a fluroescent detector. Because the fragments are in order of size, the unique fluorophores detected indicate the order of bases in the DNA sequence. |
| + | |||
| + | Sanger sequencing facilities will return two files for every reaction sequenced. The "sequencing file" which has the translated sequence ready to computationally align to the known template, and the "ab1 file" which shows a chromatograph of the sequencing. The chromatograph shows color-coded fluroescent read peaks which correspond with the ddNTP fluorophores. This raw data is then converted into the sequencing file. It is always good practice to examine the chromatograph of your sequencing data to determine the quality of the sequencing, and therefore the confidence of the called bases in your sequencing file. Today you will examine the sequencing chromatographs for quality control and align your sequencing files to determine which clone you mini-prepped contains your desired insert. | ||
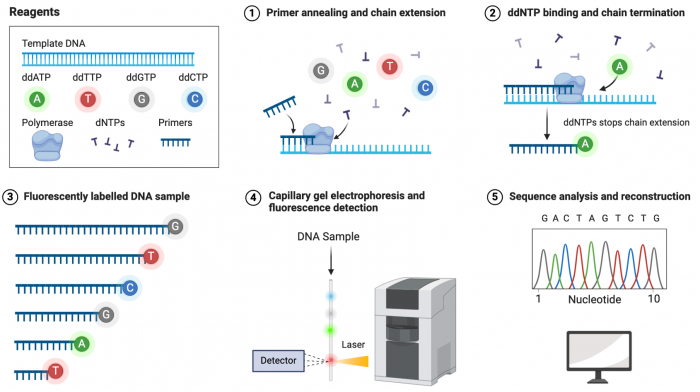
[[Image:Sp24 sanger sequencing.png|thumb|700px|center| '''Detection steps in Sanger sequencing.''' Created by Samara Ona for Biorender]] | [[Image:Sp24 sanger sequencing.png|thumb|700px|center| '''Detection steps in Sanger sequencing.''' Created by Samara Ona for Biorender]] | ||
Revision as of 21:16, 8 March 2024
Contents
Introduction
Today you will analyze data produced by Sanger sequencing of your mini-preps. In the sequencing section of the wiki yesterday, you added primers to your purified plasmids in order to amplify the section of plasmid which hopefully holds your insert. At the sequencing facility, the DNA plasmid and primer mixture was incubated with polymerase and dNTPs (deoxynucleotides). As part of a PCR reaction, the primer will bind to the complementary site on the plasmid and extend the nascent chain with base pairs, utilizing the appropriate dNTP. The sequencing facility also includes a set of fluorescently labeled ddNTPs (dideoxynucleotides). When these are incorporated, they will terminate the nascent chain because no new base can be linked. The resulting DNA fragment will end with a fluorophore corresponding with the final ddNTP base added. As the ddNTP is incorporated randomly during the extension process, the resulting pool of DNA fragments are over different sizes. These fragments are sorted by size and passed by a fluroescent detector. Because the fragments are in order of size, the unique fluorophores detected indicate the order of bases in the DNA sequence.
Sanger sequencing facilities will return two files for every reaction sequenced. The "sequencing file" which has the translated sequence ready to computationally align to the known template, and the "ab1 file" which shows a chromatograph of the sequencing. The chromatograph shows color-coded fluroescent read peaks which correspond with the ddNTP fluorophores. This raw data is then converted into the sequencing file. It is always good practice to examine the chromatograph of your sequencing data to determine the quality of the sequencing, and therefore the confidence of the called bases in your sequencing file. Today you will examine the sequencing chromatographs for quality control and align your sequencing files to determine which clone you mini-prepped contains your desired insert.
Protocols
Part 1: Examine clone sequencing results
Your goal in this section is to analyze the sequencing data for you two YSD peptide clones - two independent colonies from your cloning reaction - and then decide which colony to proceed with to engineer yeast to become a cadmium sink.
Retrieve cloning sequence results from Genewiz
- Your sequencing data is available from Genewiz. For easier access, the information was uploaded to the [20.109(S24):Class_data Class Data tab].
- Download the zip folder with your team sequencing results and confirm that there are 8 files saved in the folder.
- For each sequencing reaction, you should have one .ab1 file and one .seq file.
- Open one of the .ab1 files.
- This file contains the chromatogram for your sequencing reaction. Scroll through the sequence and ensure that the peaks are clearly defined and evenly spaced. Low signal (or peaks) or stacked peaks can provide incorrect base assignments in the sequence.
- Open one of the .seq files.
- This file contains the base assignments for your sequencing reaction. The bases are assigned by the software from the chromatogram sequence.
- The start of the a sequencing reaction result often contains several Ns, which indicates that the software was unable to assign a basepair.
In your laboratory notebook, complete the following:
- Given the chromatogram result, why might the software assign Ns in the start of the sequence?
- Visually inspect the chromatograms for all of your sequencing results.
- Do the peaks appear clearly defined or is there overlap? What might this indicate about the quality of your sequencing results?
- Do the peaks extend above the background signal? What might this indicate about the quality of your sequencing results?
Confirm insertion sequence using SnapGene
You should align your sequencing data with a known sequence, in this case the DNA sequence encoding your peptide of interest from M2D1, to identify a successful insertion. There are several web-based programs for aligning sequences and still more programs that can be purchased. The steps for using SnapGene are below. Please feel free to use any program with which you are familiar.
- Generate a new DNA file that contains the insertion sequence you generated on M2D1.
- Generate an additional new DNA file that contains the results from the sequencing reaction completed by Genewiz.
- For each sequencing result you should generate a distinct new DNA file. Remember you should have a forward and reverse sequencing result for each of your clones!
- Paste the sequence text from your sequencing run into the new DNA file window. If there were ambiguous areas of your sequencing results, these will be listed as "N" rather than "A" "T" "G" or "C" and it's fine to include Ns in the query.
- The start and end of your sequencing may have several Ns. In this case it is best to omit these Ns by pasting only the 'good' sequence that is flanked by the ambiguous sequence.
- To confirm the mutation sequence in your clones, open one of the forward sequencing results files generated in the previous step.
- Select 'Tools' --> 'Align to Reference DNA Sequence...' --> 'Align Full Sequences...' from the toolbar.
- In the window, select the file that contains the insertion oligo sequence and click 'Open'.
- A new window will open with the alignment of the two sequences. The top line of sequence shows the results of the sequencing reaction and the bottom line shows the oligo you designed.
- Are there any discrepancies or differences between the two sequences? Scroll through the entire alignment to check the full sequencing result and note any basepair changes.
- Follow the above steps to examine all of your sequencing results. Remember: you used a forward and a reverse primer to check for insertion.
- From the alignments, determine which clone has your insert.
- If both clones contain the correct sequence choose either yeast transformation to use in the rest of your experiments. If only one is correct, then this is the transformant you will use. If neither of your plasmids carry the appropriate mutation, talk to your Instructor.
In your laboratory notebook, complete the following:
- Attach a screenshot for each alignment.
- Record which clone contains the insert.
Part 2: Participate in Communication Lab workshop
Our communication instructor, Dr. Chiara Ricci-Tam, will join us today for a discussion on oral presentations. We will also have an intensive workshop on preparing for your journal article presentation as part of this extended workshop.
Reagents list
- Snapgene software
Next day: Perform flow cytometry and harvest cells to test cadmium sequestration