Difference between revisions of "20.109(S21):M3D4"
Noreen Lyell (Talk | contribs) (→Reagent list) |
Noreen Lyell (Talk | contribs) (→Protocols) |
||
| Line 12: | Line 12: | ||
==Protocols== | ==Protocols== | ||
| + | |||
| + | ===Part 2: Prepare samples for titration curve=== | ||
==== Tips for success==== | ==== Tips for success==== | ||
| Line 33: | Line 35: | ||
#Now work your way from reservoirs #2 to #12 (highest calcium concentration), and from the left-hand to the right-hand columns on your plate. Be sure to use fresh pipet tips each time! If you do contaminate a solution, let the teaching faculty know so they can put out some fresh solution. Honesty about a mistake is far preferred here to affecting every downstream experiment. | #Now work your way from reservoirs #2 to #12 (highest calcium concentration), and from the left-hand to the right-hand columns on your plate. Be sure to use fresh pipet tips each time! If you do contaminate a solution, let the teaching faculty know so they can put out some fresh solution. Honesty about a mistake is far preferred here to affecting every downstream experiment. | ||
#When you are done, alert the teaching faculty and you will be taken in small groups to measure the fluorescence values for your samples. | #When you are done, alert the teaching faculty and you will be taken in small groups to measure the fluorescence values for your samples. | ||
| + | |||
| + | ===Part 3: Fluorescence assay=== | ||
| + | |||
| + | #The BMC (BioMicro Center) has graciously agreed to let us use their plate reader. Walk over to building 68 with a member of the teaching staff. | ||
| + | #You will be shown how to set the excitation (485 nm) and emission (515 nm) wavelength on the plate reader to assay your protein. | ||
| + | #Your raw data will be posted on today's [[Talk:20.109(S16):Characterize protein expression (Day7)|Discussion page]] and emailed to you as a .txt file so you can begin your analysis. | ||
==Reagent list== | ==Reagent list== | ||
Revision as of 22:16, 2 February 2021
Contents
Introduction
As evidenced by Nagai’s work, wild-type inverse pericam is not toxic to BL21(DE3)pLysS cells. Although it is unlikely that your point mutation will dramatically change this fact, in general a novel protein may turn out to be toxic. If this is the case, only very small amounts of protein are produced before the bacteria die. Keep in mind that overexpressing a single protein may come at the expense of producing proteins needed for survival, and will most likely cause cell death eventually; however, toxic proteins hasten this demise. Aberrant toxicity can sometimes be alleviated by reducing the culture temperature (e.g., to 30 °C).
Based on its fluorescence activity, wild-type inverse pericam allows proper folding of (cp)EYFP, and based on its response to calcium, it also allows calmodulin to fold. One problem you may encounter is that your mutant proteins will no longer fold correctly. Since you made mutations in the calcium sensor part of IPC, rather than the fluorescent part, it is unlikely that your protein will destroy EYFP fluorescence. However, a common problem with misfolded proteins is the formation of insoluble aggregates, due for instance to improperly exposed hydrophobic surfaces. Proteins can be purified from these aggregates – called inclusion bodies – but the process is more labor-intensive than for soluble proteins. (The proteins must be extracted under more harsh conditions than you will use next time, then purified under denaturing conditions, before finally attempting to renature the proteins.) Inclusion bodies sometimes form simply due to very high expression of the protein of interest, causing it to pass its solubility limit. This outcome can be prevented by lowering the culture temperature, the induction duration, the amount of IPTG, or the growth phase of the bacteria.
One final point to keep in mind is that not all proteins can be produced in bacteria. Eukaryotic proteins that require post-translational modifications (such as glycosylation) for activity require eukaryotic hosts (such as yeast, or the commonly used CHO – Chinese hamster ovary – cells). Sometimes eukaryote-derived proteins will be truncated or otherwise mistranslated by E. coli due to differential codon bias; errors in translation can be prevented by providing additional tRNAs to the culture or directly to the bacteria via plasmids. Despite all this complexity, prokaryotic hosts have been plenty good enough to produce proteins for certain therapies, notably the cytokine G-CSF. This cytokine is taken by patients needing to replenish their white blood cells (e.g., after chemotherapy), and sold as Neupogen by the company Amgen.
Protocols
Part 2: Prepare samples for titration curve
Tips for success
Take great care today to limit the introduction of bubbles in your samples. When expelling fluid, pipet slowly while touching the pipet tip against the bottom or side of the well.
Protocol
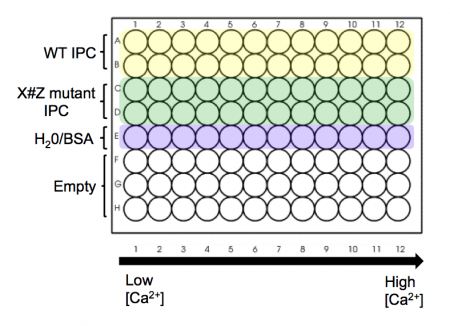
- Take a black 96-well plate, and familiarize yourself with the plate map scheme at right: top two rows are to be loaded with wild-type IPC, next two rows are to be loaded with your X#Z mutant IPC, and the final row is to be loaded with water/BSA to serve as a blank/background row.
- The dark sides of the plate reduce "cross-talk" (i.e., light leakage) between samples in adjacent wells, another potential contribution to error.
- Aliquot your wild-type protein to your plate. Use your P200 pipet to add 30 μL of protein (per well) to rows A and B of your plate.
- Aliquot you X#Z mutant IPC to your plate. Use your P200 pipet and add 30 μL of protein (per well) to rows C and D of your plate.
- Finally, add 30 μL of water with only 0.1% BSA (no IPC) to row 5(E) of your plate.
- The calcium solutions are at the front bench in shared reservoirs. Carefully carry your plate to the front bench to add these solutions with the multi-channel pipet.
- Using shared reservoir #1 (lowest calcium concentration - actually 0 nM), add 30 μL to the top five rows in the first column of the plate. Discard the pipet tips.
- Now work your way from reservoirs #2 to #12 (highest calcium concentration), and from the left-hand to the right-hand columns on your plate. Be sure to use fresh pipet tips each time! If you do contaminate a solution, let the teaching faculty know so they can put out some fresh solution. Honesty about a mistake is far preferred here to affecting every downstream experiment.
- When you are done, alert the teaching faculty and you will be taken in small groups to measure the fluorescence values for your samples.
Part 3: Fluorescence assay
- The BMC (BioMicro Center) has graciously agreed to let us use their plate reader. Walk over to building 68 with a member of the teaching staff.
- You will be shown how to set the excitation (485 nm) and emission (515 nm) wavelength on the plate reader to assay your protein.
- Your raw data will be posted on today's Discussion page and emailed to you as a .txt file so you can begin your analysis.
Reagent list
- Calcium calibration kit from Life Technologies
- Zero free calcium buffer: 10 mM EGTA in 100 mM KCl, 30 mM MOPS, pH 7.2
- 39 μM free calcium buffer: 10 mM CaEGTA in 100 mM KCl, 30 mM MOPS, pH 7.2
- Thermo Scientific Varioskan Flash Spectral Scanning Multimode Reader
Next day: Design new IPC variant