Difference between revisions of "20.109(S20):Perform qPCR experiment and continue RNA-seq data analysis (Day7)"
Noreen Lyell (Talk | contribs) (→Protocols) |
Noreen Lyell (Talk | contribs) (→Introduction) |
||
| Line 4: | Line 4: | ||
==Introduction== | ==Introduction== | ||
| + | [[Image:Screen Shot 2015-01-27 at 4.04.38 PM.png|thumb|550px|right|To eliminate clutter, the basepairs between the DNA strands were omitted. An animation of this process is linked [http://www.sigmaaldrich.com/life-science/molecular-biology/pcr/quantitative-pcr/sybr-green-based-qpcr/syber-green-animation.html here].]]Quantitative polymerase chain reaction (qPCR) allows researchers to monitor the results of PCR as amplification is occurring (this technique is also referred to as real-time polymerase chain reaction or real-time PCR). During qPCR data are collected throughout the amplification process using a fluorescent dye. The fluorescent dye is highly specific for double-stranded DNA and when bound to DNA molecules the fluorescence intensity increases proportionately to the increase in double-stranded product. In contrast, the data for traditional PCR are simply observed as a band on a gel. | ||
| + | |||
| + | As depicted in the image to the right, the fluorescent dye binds to double-stranded DNA during the cycles of PCR. At the annealing temperature the primer (blue arrow) binds to the template (black line). During an incubation at the extension temperature the new copy of DNA (orange dashed arrow) is sythesized by the polymerase enzyme. The inactive fluorescent dye molecules present in the reaction (grey stars) bind to the newly generated double-stranded DNA and become activated (green stars). | ||
| + | |||
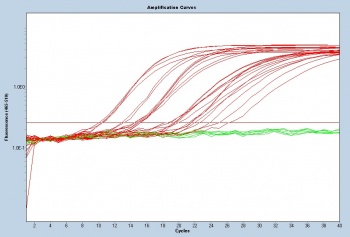
| + | [[Image:S14 M3D5 WF 18S-noiseband.jpg|thumb|350px|right|These qPCR amplification curve data were generated by Sp14 20.109ers for another experimental module!]] | ||
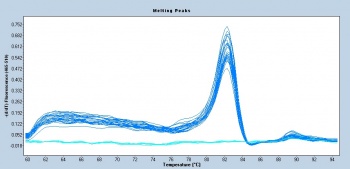
| + | [[Image:S14 M3D5 WF 18S-melt.jpg|thumb|350px|right|These qPCR melt curve data were collected by Sp14 20.109ers for another experimental module!]] | ||
| + | |||
| + | To assess gene transcript levels, you will examine the C<sub>T</sub> values from your qPCR assay. The C<sub>T</sub> values are displayed as an amplification curve following qPCR (these values are also given numerically). The initial cycles measure very little fluorescence due to low amounts of double-stranded DNA and are used to establish the inherent background fluorescence. As double-stranded product is produced, fluorescence is measured and the curve appears linear. This linear portion of the curve represents the exponential phase of PCR. Throughout the exponential phase, the curve should be smooth. Sharp points may be due to errors in reaction preparation or failures in the machine used to measure fluorescence. As mentioned previously, the first cycle in which the fluorescence measurement is above background is the C<sub>T</sub>. During the later cycles the curve shows minimal increases in fluorescence due the depletion of reagents. | ||
| + | |||
| + | Following the qPCR amplification measurements, a melt curve is completed. Melt curves assess the dissociation of double-stranded DNA while the sample is heated. As the temperature is increased, double-stranded DNA ‘melts’ as the strands dissociate. As discussed above, the fluorescent dye used in qPCR associates with double-stranded DNA and fluorescence measurements will decrease as the temperature increases. In qPCR, the melt curve is used to confirm that a single amplification product was generated during the reaction. If additional products were present, the melt curve would presumably show additional peaks. Why might this be true? Can you think of a scenario where two different products would produce a single peak in a melt curve? | ||
| + | |||
| + | <br style="clear:both;"/> | ||
==Protocols== | ==Protocols== | ||
Revision as of 20:28, 17 March 2020
Contents
Introduction
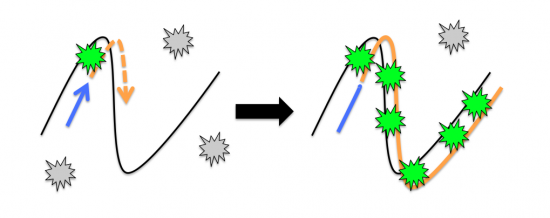
As depicted in the image to the right, the fluorescent dye binds to double-stranded DNA during the cycles of PCR. At the annealing temperature the primer (blue arrow) binds to the template (black line). During an incubation at the extension temperature the new copy of DNA (orange dashed arrow) is sythesized by the polymerase enzyme. The inactive fluorescent dye molecules present in the reaction (grey stars) bind to the newly generated double-stranded DNA and become activated (green stars).
To assess gene transcript levels, you will examine the CT values from your qPCR assay. The CT values are displayed as an amplification curve following qPCR (these values are also given numerically). The initial cycles measure very little fluorescence due to low amounts of double-stranded DNA and are used to establish the inherent background fluorescence. As double-stranded product is produced, fluorescence is measured and the curve appears linear. This linear portion of the curve represents the exponential phase of PCR. Throughout the exponential phase, the curve should be smooth. Sharp points may be due to errors in reaction preparation or failures in the machine used to measure fluorescence. As mentioned previously, the first cycle in which the fluorescence measurement is above background is the CT. During the later cycles the curve shows minimal increases in fluorescence due the depletion of reagents.
Following the qPCR amplification measurements, a melt curve is completed. Melt curves assess the dissociation of double-stranded DNA while the sample is heated. As the temperature is increased, double-stranded DNA ‘melts’ as the strands dissociate. As discussed above, the fluorescent dye used in qPCR associates with double-stranded DNA and fluorescence measurements will decrease as the temperature increases. In qPCR, the melt curve is used to confirm that a single amplification product was generated during the reaction. If additional products were present, the melt curve would presumably show additional peaks. Why might this be true? Can you think of a scenario where two different products would produce a single peak in a melt curve?
Protocols
Part 1: BE Communication Lab workshop
Our communication instructors, Dr. Sean Clarke and Dr. Prerna Bhargava, will join us today for a workshop on writing a clear and informative Research Article.
Reagents list
Next day: Examine qPCR results and complete data analysis