Difference between revisions of "Procedure: Diffusion in biological gels"
Steven Nagle (Talk | contribs) (→Making a sample Chamber) |
Steven Nagle (Talk | contribs) (→Part II: preparing the gel/particle mixtures) |
||
| Line 36: | Line 36: | ||
== Part II: preparing the gel/particle mixtures == | == Part II: preparing the gel/particle mixtures == | ||
| − | 1) Defrost a 75 µl aliquot of matrigel on ice. | + | 1) Defrost a 75 µl aliquot of matrigel on ice. (Your TA or Instructor will have an aliquot on ice if you have notified us in advance. Otherwise, you will have to wait approximately 45 minutes for the gel to slowly deforst, on ice. They cannot be quickly defrosted, as this may cause gelation around the edges of the tube.). |
| − | 2) Once the gel has thawed, | + | 2) Once the gel has thawed, place a different 1 uL of your analytes in each of three eppendorf tubes: use neutral liposomes in the first, positively charged liposomes for the second, and the polystyrene particles to the third. |
| − | 3) | + | 3) Label your test tubes accordingly to keep track of your sample mixtures! Include your group number on the tubes. |
| − | + | ||
| − | + | Keep all your aliquots on ice! | |
| − | + | ||
| − | + | 4) Now place 25 µl DMEM in the two tubes containing the liposomes, and 25 µl of your group's salt solution into the third tube with the nanoparticles. Mix by gentle pipetting, avoid creating bubbles! (Be aware, once you add the gel, bubbles will form easily so mix carefully then, and do not "push out" the last of the gel solution.) | |
| − | + | 5) Finally, distribute 25 µl matrigel into each of your three tubes. | |
| − | 7) Your hydrogel samples are now ready for microscopy. | + | ''' NEVER vortex the gel solution! ''' |
| + | |||
| + | Keep all your aliquotes on ice! | ||
| + | |||
| + | You should now have three aliquots containing 51 µl of a gel/particle mixture each. Again, make sure they are labeled. | ||
| + | |||
| + | 5) Fill each of your sample chambers with a different sample and, if you haven't already done so, label the glass slides accordingly. Include your group number. | ||
| + | |||
| + | 6) Now place all of your filled sample chambers for 20 minutes in the incubator at 37°C to induce gelation. | ||
| + | |||
| + | 7) Place the slides on your vibration table to cool for maybe a minute. Cover them with foil or a box top to keep them from bleaching. | ||
| + | |||
| + | 8) Your hydrogel samples are now ready for microscopy. | ||
{{Template:20.309 bottom}} | {{Template:20.309 bottom}} | ||
Revision as of 01:32, 5 October 2010
| |
This procedure uses sensitive biological samples. Wear gloves to avoid contamination. |
Summary
You are going to test the diffusion behavior of three different particle types in matrigel. The three particle species are:
a) 1 µm liposomes, positively charged
b) 1 µm liposomes, neutral
c) 500 nm polystyrene beads, positively charged
Each of these analytes are prepared for you in tubes and will be kept on ice any day someone says they are going to do the particle tracking.
Making a sample Chamber
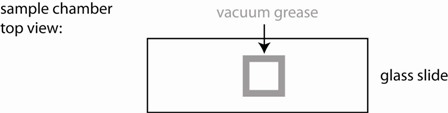
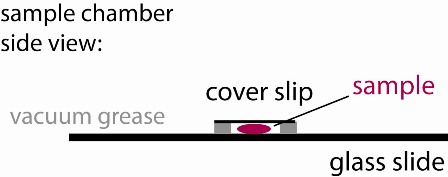
1) Use the vacuum grease filled syringe to place an open ~ 15x15 mm square on the middle of a glass slide. Make sure it is small enough so that it can be fully covered with a small cover slip. Ensure that the linings are continuous (see figure), otherwise your sample chamber will leak and you will have lots of drift.
1a) You will need to hold on to the base of the tip on the syringe, as it will tend to unscrew. If it does, simply screw it back in and hold on tigher.
2) Once it is prepared below, place ~30 µl of your sample into the vacuum grease well of the sample chamber you crafted above.
3) Label the glass slide to keep track of your sample. Be precise because your samples may last a few days if you keep them wrapped in foil.
4) Cover the well with a small cover slip creating a sandwich (see figure). Press only enough to make contact with the sample and provide a continuous seal around the perimeter of your well. If some fluid leaks out, that is OK, just gently slide the cover slip around laterally to better ensure a continuous seal. To check, look around the perimeter at a glancing angle, using the bench ligths for illuination.
Part II: preparing the gel/particle mixtures
1) Defrost a 75 µl aliquot of matrigel on ice. (Your TA or Instructor will have an aliquot on ice if you have notified us in advance. Otherwise, you will have to wait approximately 45 minutes for the gel to slowly deforst, on ice. They cannot be quickly defrosted, as this may cause gelation around the edges of the tube.).
2) Once the gel has thawed, place a different 1 uL of your analytes in each of three eppendorf tubes: use neutral liposomes in the first, positively charged liposomes for the second, and the polystyrene particles to the third.
3) Label your test tubes accordingly to keep track of your sample mixtures! Include your group number on the tubes.
Keep all your aliquots on ice!
4) Now place 25 µl DMEM in the two tubes containing the liposomes, and 25 µl of your group's salt solution into the third tube with the nanoparticles. Mix by gentle pipetting, avoid creating bubbles! (Be aware, once you add the gel, bubbles will form easily so mix carefully then, and do not "push out" the last of the gel solution.)
5) Finally, distribute 25 µl matrigel into each of your three tubes.
NEVER vortex the gel solution!
Keep all your aliquotes on ice!
You should now have three aliquots containing 51 µl of a gel/particle mixture each. Again, make sure they are labeled.
5) Fill each of your sample chambers with a different sample and, if you haven't already done so, label the glass slides accordingly. Include your group number.
6) Now place all of your filled sample chambers for 20 minutes in the incubator at 37°C to induce gelation.
7) Place the slides on your vibration table to cool for maybe a minute. Cover them with foil or a box top to keep them from bleaching.
8) Your hydrogel samples are now ready for microscopy.