Difference between revisions of "20.109(F22):M1D4"
Becky Meyer (Talk | contribs) (→Part 1: Analyze γH2AX images by counting foci) |
Noreen Lyell (Talk | contribs) (→Protocols) |
||
| Line 73: | Line 73: | ||
<font color = #4a9152 >'''In your laboratory notebook,'''</font color> complete the following: | <font color = #4a9152 >'''In your laboratory notebook,'''</font color> complete the following: | ||
*Based on your reading and the group discussion of the article, answer the questions above. | *Based on your reading and the group discussion of the article, answer the questions above. | ||
| + | |||
| + | ===Part 3: Learn about the CometChip=== | ||
| + | |||
| + | As discussed in the prelab lecture, you will use two methods to assess DNA damage: the γH2AX assay and the CometChip. Before we review the CometChip experimental details, it is best to familiarize yourself with the procedure and assay. | ||
| + | |||
| + | Read the Abstract and Introduction in the following publication: | ||
| + | |||
| + | [[Media:CometChip Jove article.pdf| CometChip: A high-throughput 96-well platform for measuring DNA damage in microarrayed human cells.]] ''Journal of Visualized Experiments.'' (2014) 92: 1-11. | ||
| + | |||
| + | <font color = #4a9152 >'''In your laboratory notebook,'''</font color> answer the following questions: | ||
| + | *Why is it important to study DNA damage? | ||
| + | **You can consider the information provided in lecture / prelab to answer this question. | ||
| + | *How does the CometChip estimate the level of DNA damage within cells? | ||
| + | *List two issues / problems with the comet assay? | ||
| + | *List two improvements provided by the CometChip assay? | ||
| + | |||
| + | ===Part 4: Prepare CometChip=== | ||
| + | |||
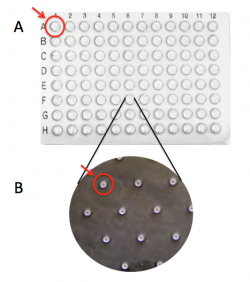
| + | [[Image:Fa18 M1D2 macrowell vs microwell.png|thumb|250px|right|'''Image of CometChip''' showing distinction between (A) 'macrowell' and (B) 'microwell'.]]The CometChip is simply a thin layer of agarose with microwells. It is important to differentiate between the terms 'macrowell' and 'microwell' for your experiments. A bottomless 96-well plate is placed on top of the agarose CometChip to create the macrowells for the CometChip assay (panel A). This enables researchers to control which cells are exposed to which treatment. The microwells were stamped into the agarose when you made your CometChip (panel B). Within each well are ~ 300 microwells, which are ~40 μm in diameter and 40 μm in depth. | ||
| + | |||
| + | <font color = #0d368e>'''To ensure the steps required for preparing a CometChip are clear, the Instructor will provide a live demonstration of this process. You should provide a written description of the procedure in your laboratory notebook!'''</font color> | ||
| + | |||
| + | <font color = #4a9152 >'''In your laboratory notebook,'''</font color> complete the following: | ||
| + | *Provide a written overview / description of the the procedure used to prepare a CometChip (from the live demonstration). | ||
| + | *Onto which side of the GelBond is the agarose poured to make a CometChip? | ||
| + | *How are the microwells generated in the agarose of the CometChip? | ||
| + | |||
| + | In addition, a video demonstrating the finishing procedure to make a CometChip is linked here: [https://www.dropbox.com/s/nufmtrashmu4160/%20Finishing%20the%20CometChip.mp4?dl=0 Making the CometChip] | ||
| + | |||
| + | <br style="clear:both" /> | ||
==Reagents list== | ==Reagents list== | ||
Revision as of 15:38, 7 September 2022
Contents
Introduction
Protocols
Part 1: Analyze γH2AX images by counting foci
To analyze your data, you will use ImageJ and a plugin written by Joshua Corrigan to enumerate the γH2AX foci present in the nuclei of the treated cells. If you would like to review concepts used in this code, please review a protocol written by researchers at Duke University outlined here.
In this analysis, you will calculate the average foci per nuclei, indicating DNA strand breaks (γH2AX staining). Please note that this process is not automated, so each image will have to be analyzed individually.
- To begin, ensure that all of your H2AX images are in an easily accessible location.
- Download the quantification plugin here.
- Open ImageJ and go to Analyze -> Set Measurements
- In the Set Measurements window, make sure the following boxes are checked: Area, Mean gray value, Min & max gray value, Shape descriptors, Integrated density, Display label
- Drag the downloaded plugin into ImageJ to open the macro window.
- Open an image in ImageJ.
- Click on the "Run" button in the macro window to begin analysis.
- This will separate the stacks of the multichannel image and allow you to begin the thresholding process.
- Manually set the threshold so that the nuclei are solid red shapes reflecting the DAPI signal parameters in the image.
- Make sure the cell nuclei are highlighted in red.
- Adjust the threshold values to properly identify the majority of the cells' nuclei.
- Click "ok" to begin the process of watershedding the nuclei (defining the boundaries and separating nuclei in close proximity). The macro will also use the presence of the DAPI signal to create a "mask" for use in the FITC channel.
- The program will switch to the image of the FITC channel to examine γH2AX sites. It will also turn up the brightness in this channel to allow foci to be more easily identified.
- You will now use the FITC channel to set a threshold level to identify foci.
- In the next window, choose the folder containing your .tif image stacks for the chosen condition, and click "Open."
- You will be prompted to "Enter result file name" to name your spreadsheet for this analysis. Do so, and click "Ok".
- In the dialog box titled "Choose Intensity Threshold Values," type in all the corresponding threshold values you have chosen, and click "OK."
- Please wait for the script to run through all your images. In the end all the image files will pop up, along with the "drawings" that show where it identified cells in your images
- On the "Drawing" windows that popped up, similar to the images shown here, each area identified by your fluorescence thresholds are assigned a number. Each of those numbers are listed in the corresponding Excel spreadsheets, followed by the information assessed by the ImageJ script.
- For the DAPI channel, most of the identified regions correspond to a single nuclei. For the FITC channel, one may argue that for a cell having a significant amount of double strand breaks, the entire nucleus (rather than a few spots) shows up as being identified by the intensity thresholds provided.
- The script will output resulting Excel files into your image folder. The Excel files contain raw data of each region of interest identified by the fluorescence intensity thresholds you chose.
- You may use the Excel files to assess how foci are identified in each nuclei. To do this, divide the Raw Integrated Density (RawIntDen) for each nuclei by 255 (each maxima representing a foci has a value of 255) to identify the number of foci in each nucleus. You can now average the number for each image in the condition.
- Repeat these steps for the additional conditions to quantify your results.
In your laboratory notebook, complete the following:
- How does the data obtained in the two analysis approaches compare? Are the results the same? Different?
- Which analysis approach best represents the raw data images? Why?
Part 2: Participate in group paper discussion
To further help you in preparing your Data summary, we will discuss how similar data are presented in a publication from the Engelward laboratory.
Weingeist, D. M., et al. "Single-cell microarray enables high-throughput evaulation of DNA double-strand breaks and DNA repair inhibitors." Cell Cycle. (2013) 126:907-915.
From the Introduction
Consider the key components of an introduction:
- What is the big picture?
- Is the importance of this research clear?
- Are you provided with the information you need to understand the research?
- Do the authors include a preview of the key results?
From the Results
Carefully examine the figures. First, read the captions and use the information to 'interpret' the data presented within the image. Second, read the text within the results section that describes the figure.
- Do you agree with the conclusion(s) reached by the authors?
- What controls were included and are they appropriate for the experiment performed?
- Are you convinced that the data are accurate and/or representative?
From the Discussion
Consider the following components of a discussion:
- Are the results summarized?
- Did the authors 'tie' the data together into a cohesive and well-interpreted story?
- Do the authors overreach when interpreting the data?
- Are the data linked back to the big picture from the introduction?
In your laboratory notebook, complete the following:
- Based on your reading and the group discussion of the article, answer the questions above.
Part 3: Learn about the CometChip
As discussed in the prelab lecture, you will use two methods to assess DNA damage: the γH2AX assay and the CometChip. Before we review the CometChip experimental details, it is best to familiarize yourself with the procedure and assay.
Read the Abstract and Introduction in the following publication:
CometChip: A high-throughput 96-well platform for measuring DNA damage in microarrayed human cells. Journal of Visualized Experiments. (2014) 92: 1-11.
In your laboratory notebook, answer the following questions:
- Why is it important to study DNA damage?
- You can consider the information provided in lecture / prelab to answer this question.
- How does the CometChip estimate the level of DNA damage within cells?
- List two issues / problems with the comet assay?
- List two improvements provided by the CometChip assay?
Part 4: Prepare CometChip
The CometChip is simply a thin layer of agarose with microwells. It is important to differentiate between the terms 'macrowell' and 'microwell' for your experiments. A bottomless 96-well plate is placed on top of the agarose CometChip to create the macrowells for the CometChip assay (panel A). This enables researchers to control which cells are exposed to which treatment. The microwells were stamped into the agarose when you made your CometChip (panel B). Within each well are ~ 300 microwells, which are ~40 μm in diameter and 40 μm in depth.To ensure the steps required for preparing a CometChip are clear, the Instructor will provide a live demonstration of this process. You should provide a written description of the procedure in your laboratory notebook!
In your laboratory notebook, complete the following:
- Provide a written overview / description of the the procedure used to prepare a CometChip (from the live demonstration).
- Onto which side of the GelBond is the agarose poured to make a CometChip?
- How are the microwells generated in the agarose of the CometChip?
In addition, a video demonstrating the finishing procedure to make a CometChip is linked here: Making the CometChip
Reagents list
Next day: Treat cells for CometChip assay