Difference between revisions of "20.109(S07): Measuring calcium in vitro"
| Line 2: | Line 2: | ||
==Introduction== | ==Introduction== | ||
| + | When we learned about M13, we saw how cleverly this virus interacts with its bacterial host, for example using a natural and common bacterial structure to gain access to the cell and then harnessing the cell’s transcription and translation machinery to make more viral particles. What we did not consider was the bacterial response to the viral attack. In fact, bacteria do “notice” viral infection and mount an SOS response within minutes. Stress response genes are up-regulated in an effort to protect the bacterial host from far more severe consequences of phage infection like lysis or destruction of it’s own genome. | ||
| + | |||
| + | In this next module we will turn our attention from the stress-or to the stress-ee. and consider the signals that go on inside cells to help them understand their world. It’s a complex world and so not surprisingly there exist an impressive volume and diversity of cellular messengers. Some relay information between a cell’s outer membrane and its internal processors. Messages in eukaryotic cells also travel between the nucleus and the cytoplasm, plus between and within organelles. Signaling information is transmitted through covalent modifications of the cell’s macromolecules. Common modifications include phosphate-, methyl-, and acetyl- groups, as well as peptide tags to target proteins for particular cellular locations or for degradation. The cellular signal we will consider for the next experimental module is calcium, which is not covalently added to proteins but rather modulates the shape of the proteins that bind it. | ||
| + | |||
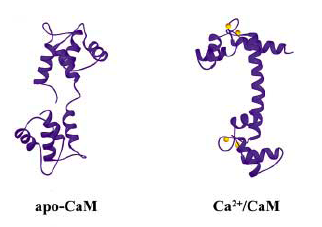
| + | Calcium is the most abundant mineral in our body yet the concentration of Ca2+ within our cells is extremely low (~10^-7M). Calcium pumps and gated channels work hard to establish and keep this steep gradient. Inside the cell, calcium is spatially regulated (restricted to some organelles and not others), poised for release when appropriate. Calcium-handling proteins are a large and widely distributed family of proteins. Many share a characteristic dumbbell shape, with two globular domains connected by a flexible linker region. Calmodulin, the calcium sensing protein whose structure you examined last time, binds four Ca2+ ions, two with high affinity and two with low affinity. The high affinity sites can be filled at low calcium concentrations, but when calcium gets released from intracellular stores, the low affinity sites are also filled, inducing a large conformational change in the protein. These expose non-polar regions of calmodulin that can bind to non-polar regions of a target molecule. Notice how the backbone of calmodulin extends in the presence of calcium (figure from [http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?db=pubmed&cmd=Retrieve&dopt=AbstractPlus&list_uids=12542690&query_hl=6&itool=pubmed_docsum the review by Vetter and LeClerc, FEBS 2003]) <br> | ||
| + | [[Image:Macintosh HD-Users-nkuldell-Desktop-BE109(S07)-S07 class specific info-wiki art-apo vs Ca bound from FEBS.png|700pxls|center| calmodulin's shape with (right) and without (left) Ca2+, from FEBS 2003]] | ||
| + | |||
| + | An interesting example of this is seen with anthrax toxin, which exploits both the cellular distribution of calmodulin as well as its mechanism for calcium sensing. One component of the anthrax toxin, called EF, can bind to calmodulin in the “low calcium” conformation, twisting the linker so it cannot respond to high calcium concentrations, effectively blocking its natural signaling functions. With calmodulin out of the picture, the cells have no way to convert the cAMP formed by EF back into ATP, leading to depletion of the cell’s energy stores. This mechanism seems particularly clever when you remember that calcium-sensing motifs are widespread and abundant but only in animal cells. Thus the Bacillus anthracis bacteria don’t put deplete their own energy stores when anthrax toxin is expressed. | ||
| + | |||
| + | Today we will measure calcium concentrations of several solutions. Beyond learning some important laboratory techniques (like standard curves and spectrophotometric analysis of chromogenic reactions), today’s work should emphasize the limited sensitivity and range of such techniques. Luckily cells are better at sensing calcium, as we’ll see when we look at the genetically encoded calcium sensor next week. | ||
| + | |||
==Protocols== | ==Protocols== | ||
===Part 1: Ca++ measurements=== | ===Part 1: Ca++ measurements=== | ||
Revision as of 21:48, 26 December 2006
Contents
Introduction
When we learned about M13, we saw how cleverly this virus interacts with its bacterial host, for example using a natural and common bacterial structure to gain access to the cell and then harnessing the cell’s transcription and translation machinery to make more viral particles. What we did not consider was the bacterial response to the viral attack. In fact, bacteria do “notice” viral infection and mount an SOS response within minutes. Stress response genes are up-regulated in an effort to protect the bacterial host from far more severe consequences of phage infection like lysis or destruction of it’s own genome.
In this next module we will turn our attention from the stress-or to the stress-ee. and consider the signals that go on inside cells to help them understand their world. It’s a complex world and so not surprisingly there exist an impressive volume and diversity of cellular messengers. Some relay information between a cell’s outer membrane and its internal processors. Messages in eukaryotic cells also travel between the nucleus and the cytoplasm, plus between and within organelles. Signaling information is transmitted through covalent modifications of the cell’s macromolecules. Common modifications include phosphate-, methyl-, and acetyl- groups, as well as peptide tags to target proteins for particular cellular locations or for degradation. The cellular signal we will consider for the next experimental module is calcium, which is not covalently added to proteins but rather modulates the shape of the proteins that bind it.
Calcium is the most abundant mineral in our body yet the concentration of Ca2+ within our cells is extremely low (~10^-7M). Calcium pumps and gated channels work hard to establish and keep this steep gradient. Inside the cell, calcium is spatially regulated (restricted to some organelles and not others), poised for release when appropriate. Calcium-handling proteins are a large and widely distributed family of proteins. Many share a characteristic dumbbell shape, with two globular domains connected by a flexible linker region. Calmodulin, the calcium sensing protein whose structure you examined last time, binds four Ca2+ ions, two with high affinity and two with low affinity. The high affinity sites can be filled at low calcium concentrations, but when calcium gets released from intracellular stores, the low affinity sites are also filled, inducing a large conformational change in the protein. These expose non-polar regions of calmodulin that can bind to non-polar regions of a target molecule. Notice how the backbone of calmodulin extends in the presence of calcium (figure from the review by Vetter and LeClerc, FEBS 2003)
An interesting example of this is seen with anthrax toxin, which exploits both the cellular distribution of calmodulin as well as its mechanism for calcium sensing. One component of the anthrax toxin, called EF, can bind to calmodulin in the “low calcium” conformation, twisting the linker so it cannot respond to high calcium concentrations, effectively blocking its natural signaling functions. With calmodulin out of the picture, the cells have no way to convert the cAMP formed by EF back into ATP, leading to depletion of the cell’s energy stores. This mechanism seems particularly clever when you remember that calcium-sensing motifs are widespread and abundant but only in animal cells. Thus the Bacillus anthracis bacteria don’t put deplete their own energy stores when anthrax toxin is expressed.
Today we will measure calcium concentrations of several solutions. Beyond learning some important laboratory techniques (like standard curves and spectrophotometric analysis of chromogenic reactions), today’s work should emphasize the limited sensitivity and range of such techniques. Luckily cells are better at sensing calcium, as we’ll see when we look at the genetically encoded calcium sensor next week.
Protocols
Part 1: Ca++ measurements
- compare calcium diagnostic test to water test kit
Part 2: Cell culture
- count cells
DONE!