20.109(S19):Practice tissue culture techniques and prepare cells for RNA purification (Day1)
Contents
Introduction
We will use parental DLD-1 and mutant DLD-1 BRCA2-/- cell lines in this module to examine gene expression in cancer cells. The parental DLD-1 cell line originated from the colon of a male patient with colorectal adenocarcinoma Dukes Type C. The BRCA2-/- mutant cell line was created via disruption of BRCA2 exon 11. This mutation results in cells that are deficient in DNA repair. In the image to the right, cell survival decreased as higher concentrations of a DNA damaging agent, etoposide, were added.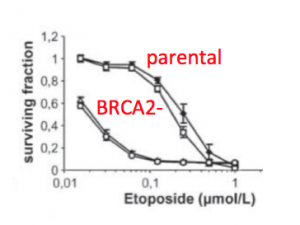
You will work with these cells, mostly, in our tissue culture room. Tissue culture was developed ~100 years ago as a means to study mammalian biology, and since that time we have learned a tremendous amount by observing the behavior of mammalian cells maintained in the laboratory. The term tissue culture was originally coined because researchers were doing exactly that, extracting tissue and letting it live in a dish for a short time. Today, most tissue culture experiments are done using cells rather than tissues. Much of what we know about cancer, heritable diseases, and the effects of the environment on human health has been derived from studies of cultured cells.
What types of cells do people study, and from where do they come? Cells acquired directly from animal tissue are called primary cells. They are difficult to culture, largely because primary cells in this context divide only a limited number of times. This limitation on the lifespan of cultured primary cells, called the Hayflick limit, is a problem because it requires a researcher to repeatedly remove tissues from animals to complete a study. Cell isolation processes can be quite labor-intensive, and also can complicate data analysis due to inherent animal-to-animal variation. To get around this problem, researchers study cells that are immortal, which means the cells are able to divide indefinitely. Though using immortal cells is preferable for many reasons, some inherent cell-to-cell variation still exists in such populations and the genetic changes that cause immortality may affect experimental outcomes.
The art of tissue culture lies in the ability to create conditions that are similar to what a cell would experience in an animal, namely 37°C and neutral pH. Blood nourishes the cells in an animal, and blood components are therefore used to feed cells in culture. Serum, the cell-free (and clotting-factor free) component of blood, contains many of the factors necessary to support the growth of cells outside the animal. Consequently, serum is frequently added to tissue culture medium, although serum-free media (also called chemically defined media) exist and support some types of cultured cells.
Cultured mammalian cells must grow in a germ-free environment and researchers using tissue culture must be skilled in sterile or aseptic technique. Bacteria double very quickly relative to mammalian cells. An average mammalian cell doubles about once per day whereas many common bacteria can double every 20 minutes under optimal conditions. Consequently, if you were to co-culture 100 mammalian cells and 1 bacteria together in a flask, within 24 hours you would have ~200 unhappy mammalian cells, and about 100 million happy bacteria! Needless to say, you would not find it very useful to continue to study the behavior of your mammalian cells under these conditions.
Protocols
Part 1: Practice tissue culture techniques
As you prepare to work in the tissue culture room, review the following resource:
Part 1a: Demonstration of best practices
The teaching faculty will show you several key techniques that will be important to your success in tissue culture. Please carefully observe the following procedures, take notes, and ask questions!
- Prepare the tissue culture hood.
- Observe your cells.
- Trypsinization of cultured cells.
- Calculate cell culture density.
- Seed cells.
- Clean tissue culture hood.
Part 1b: Seed cells for RNA purification
You will use the techniques you learned during the demonstration to seed T75 flasks with cells. These cells will be used to assess the effect of DNA damage on gene expression. More specifically, you will examine this effect in the BRCA2-/- mutant cells compared to the DLD-1 parental cells.
Each team will receive two T25 culture flasks: one with DLD-1 (parental) cells, and one with BRCA2-/- mutant cells. You will enzymatically detach these adherent cells, count them, and seed them at specific densities into fresh T25 flasks to prevent the cells from overgrowing.
It is essential that you do not mix up or cross-contaminate the DLD-1 and BRCA2-/- cell lines! We suggest that one partner is responsible for each flask, and that partners do not share Pasteur pipets or other equipment.
Preparing tissue culture hood
- The tissue culture hood is partly set up for you. Finish preparing your hood according to the demonstration, first bringing in any remaining supplies you will need, then obtaining the pre-warmed reagents from the water bath, and finally retrieving your cells from the 37 °C incubator.
- Don't forget to spray everything down with 70% ethanol!
- One of the greatest sources for tissue culture contamination is moving materials in and out of the hood because this disturbs the air flow that maintains a sterile environment inside the hood. Think about what you will need during your experiment to avoid moving your arms in and out of the hood while you are handling your cells.
Collecting cells
- Examine your cell cultures as you remove the flasks from the incubator.
- Look first at the color and clarity of the media. Fresh media is reddish-orange in color and if the media in your flask is yellow or cloudy, it could mean that the cells are overgrown, contaminated, or starved for CO2.
- Next, look at the cells using the inverted microscope. Note their shape, arrangement, and how densely the cells cover the surface of the flask.
- After you look at your cells, take the flask to your tissue culture hood to begin the seeding procedure.
- Aspirate the media from the cells using a sterile Pasteur pipet.
- Wash the cells by adding 2 mL PBS using a 5 mL pipet. Slightly tip the flask back and forth to rinse the cells then aspirate the PBS with a fresh Pasteur pipet.
- To dislodge the cells from the flask, you will add trypsin, a proteolytic enzyme.
- With a 2 mL pipet, add 1 mL of trypsin to the flask.
- 2 mL pipets are tricky! They fill up quickly. Be careful not to pull up the liquid too quickly or it will go all the way up your pipet into the pipet-aid! If this happens, please alert the teaching faculty rather than returning the pipet-aid to the rack.
- Tip the flask in each direction to distribute the trypsin evenly then incubate the cells at 37°C for 3 minutes using a timer.
- This is a great time to clear out your trash and read ahead!
- Retrieve your flasks from the incubator and firmly tap the bottom 10 times to dislodge the cells.
- Check your cells using the microscope to ensure they are dislodged. They should appear round and move freely.
- If your cells are not detached from the flask, incubate at 37 °C for an additional minute.
- When your cells are dislodged, move your flask back into the tissue culture hood and add 3 mL of media to the cells then pipet the liquid up and down (“triturate”) to break up cells that are clumped together and suspend them in the liquid.
- Note: do not take up or release all the liquid, in order to avoid bubbles.
- Transfer the suspended cells into a labeled 15 mL conical tube
- Transfer 90 μL of your cell suspension from the 15 mL conical tube into a labeled eppendorf tube.
- Be sure to cap your conical tube and eppendorf tube after you transfer your cells.
Counting cells
- Remove the eppendorf with your cells from the tissue culture hood.
- Add 10 μL of Trypan blue to the eppendorf tube and pipet up and down to mix.
- With a new pipet tip, carefully pipet 10 μL of the stained cells between the hemocytometer and (weighted) glass cover slip, as shown to you by the teaching faculty.
- Keep BRCA2-/- on the 'top' and DLD-1 on the 'bottom', so you don't forget which is which.
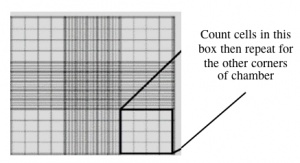
- Count the cells that fall within the four corner squares (with a 4x4 etched grid pattern), average (i.e. divide by 4), and then multiply by 10,000 to determine the number of cells/mL.
Seeding cells
- Obtain two T25 flasks and label one DLD-1 and one BRCA2-/-.
- Include your section information and the date.
- Calculate the volume of each cell suspension you need to seed 250,000 DLD-1 cells and 500,000 BRCA2-/- cells per T25 flask.
- For example, if your concentration is 1 million (1 M) cells/mL, you would seed one fresh T25 flasks with 250 μL of DLD-1 cells.
- Subtract the volume of cell suspension that you calculated in Step #2 from 5 mL.
- Add this volume of media to the fresh T25 flask.
- Note: this volume will likely be different for the DLD-1 and BRCA2-/- flasks.
- Add the volume of cell suspension that you calculated in Step #2.
- You should mix your cell suspension by pipetting before distributing the small volume of cells to the flasks as the cells likely settled to the bottom of the conical while you were counting.
- Finally, tilt your flasks back and forth to distribute the cells evenly.
- Before moving your flasks to the 37 °C incubator, use the microscope to visually confirm that cells are present.
The teaching faculty will use your cultures to seed larger flasks, T75 flasks, for the experiments to be completed in the subsequent laboratory sessions.
Cleaning the tissue culture hood
The next group who uses your hood should find the surfaces wiped down and free of equipment.
- Aspirate any remaining cell suspensions.
- Dispose of all vessels that held cells in the biohazard waste box and be sure that all sharps are in the sharps jar.
- Remove any equipment or supplies that you transferred into the hood and return to the appropriate location.
- Please leave the equipment that was already there.
- Spray the TC hood surface with 70% ethanol and wipe with paper towels.
- Be sure the paper towels are disposed of in the biohazard waste box!
- Empty the benchtop biohazard bucket into the biohazard waste box.
Part 2: Research tissue culture cell lines
In this module you will assess gene expression in DLD-1 parental and BRCA2-/- mutant cells. In this exercise, you will learn more about these cell lines to prepare you for your experiments and your M2 Research article.
The parental strain is DLD-1, and an important resource for learning about common cell lines is the American Type Culture Collection, or ATCC. Visit the DLD-1 homepage and answer the questions below in your laboratory notebook.
- From what source were these cells derived (organism and tissue)? What other details concerning the host are provided?
- Do these cells float in the culture media, or stick to the culture dish? How do you know?
- What is the morphology of these cells? What does this mean?
- Carefully read through the Culture Methods provided for these cells. Does this procedure differ from what you did in the previous laboratory session? How?
The BRCA2-/- mutant strain was generated via targeted disruption of BRCA2 exon 11 using recombinant adeno-associated virus gene editing technology. DLD-1 and BRCA2-/- are isogenic cell lines.
- Provide a brief description of how adeno-associated virus gene editing technology disrupts genes to generate mutant cell lines.
- What does it mean when cell lines are isogenic? Why is this useful in research?
Lastly, to provide context for your research you will review the following journal article concerning the BRCA2-/- cell line and answer the questions below.
- A syngeneic variance library for functional annotation of human variation: application to BRCA2. (2008) Cancer Res by Hucl et al.
- Read the Introduction. Given the information provided here and in lecture, why was exon 11 targeted to generate the BRCA2-/2 mutant (ie why does this result in a BRCA2 null mutant)?
- Review Figure 2 and the following results sub-sections: RAD51 focus formation, Chromosomal instability, Cell survival after irradiation and Cell proliferation on drug treatment.
- The authors report that RAD51 focus formation and chromosomal aberrations are decreased and increased, respectively in BRCA2-/- cells compared to DLD-1 cells after treatment with a DNA-damaging agent. What are the implications of this in disease, specifically why might these outcomes result in higher rates of cancer in BRCA2 null individuals?
- Cell survival following irradiation and various drug treatments are reported in the results. Given the data, which treatment is better suited to treat patients with BRCA2 null cancer? Use what you know about the role of BRCA2 and the type of DNA damage induced by the treatment agent to support your answer.
- Write a hypothesis that states your expectation(s) related to cell survival for the conditions you will test: DLD-1 and BRCA2-/- with no treatment, with etoposide, and with etoposide plus increasing drug concentration.
Reagents
From Thermo Fisher:
- DLD-1 parental cells
- BRCA2-/- mutant cells
Media components from Life Technologies, Inc. (unless noted otherwise):
- DLD-1 and BRCA2-/- cell media
- RPMI 1640
- 10% fetal bovine serum (FBS)
- 100X antibiotic solution from Cellgro
- 10,000 U/mL Penicillin
- 10,000 U/mL Streptomycin
- phosphate-buffered saline (PBS)
- 0.05% trypsin/0.91 mM EDTA
- trypan blue (Sigma)
- Incubator maintains 37°C, 5% CO2 and 95% relative humidity
Next day: Etoposide treat cells for RNA purification
Previous day: Complete data analysis