20.109(S17):Purification of induced protein (Day2)
Contents
Introduction
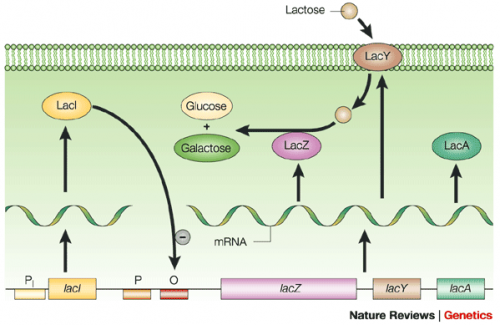
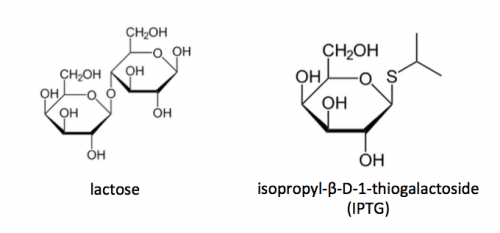
Last time you used the lactose-analogue isopropyl β-D-1-thiogalactopyranoside (IPTG) to induce expression of FKBP12 in BL21(DE3)pLysS bacteria. The use of IPTG to induce protein expression is based on the native lac operon used for lactose metabolism in bacterial cells.
The lac operon is composed of four genes: lacI, lacZ, lacY, and lacA. When lactose is absent, LacI (the protein encoded by lacI) binds to the operator sequence (O) upstream of lacZYA. In the presence of lactose, LacI and lactose form a complex which relieves repression of lacZYA transcription. LacZ is a β-galactosidase that cleaves lactose resulting in glucose and galactose. LacY, a β-galactoside permease, facilitates the transport of lactose across the cell membrane, and LacA, a β-galactoside transacetylase, transfers an acetyl group from acetyl-CoA to β-galactosides.The native lac operon is a powerful tool in engineering protein expression systems because it enables researchers to control gene expression using inducer molecules. The lacZYA genes are only expressed when lactose is present. If a gene of interest is cloned downstream of the operator sequence, the expression of this gene can be controlled by LacI repression and lactose derepression. To further control the system for protein expression, IPTG is used as a lactose-analog as it is not metabolized by the cells.
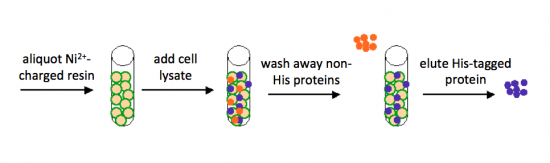
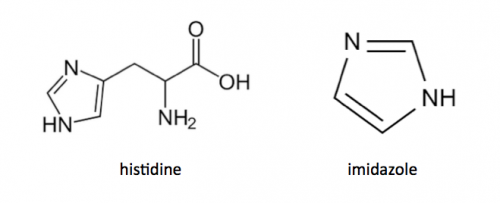
Today you will isolate your FKBP12 protein from the bacterial cells. The bacterial expression vector we are using (pRSETb) contains six histidine codons downstream of a bacterial promoter and in-frame with a start codon. Our resultant protein is therefore marked by the presence of these additional encoded residues, or His-tagged. Histidine has several interesting properties, notably its near-neutral pKa, and His-rich peptides are promiscuous binders, particularly to metals. (For example, histidine side chains help coordinate iron molecules in hemoglobin.)
You will use a nickel-agarose resin to separate your protein of interest (FKPB12) from the other proteins present in the bacteria. The His-tagged protein will preferentially bind to the nickel-coated beads, while proteins irrelevant to our purposes in Module 1 can be washed away. Remember, the BL21(DE3)pLysS cells are not only producing your protein, but also the proteins needed for cellular function and survival. Finally, a high concentration of imidazole (which is the side chain of histidine) can be used to elute the His-tagged FKBP12 by competition.
Protocols
Part 1: Lyse BL21(DE3)pLysS pRSETb_FKBP12 cells
- Retrieve your BL21(DE3)pLysS pRSETb_FKBP12 cell pellet from the front laboratory bench and leave it on your bench to thaw.
- You will also obtain a cell pellet of uninduced cells that was prepared by the teaching faculty as a control to examine the IPTG induction step.
- Prepare 3 mL of lysis buffer.
- Use the information in the table below to calculate the volume of each stock reagent that is needed to prepare the lysis buffer with the specified final concentrations.
- Confirm your calculations with the teaching faculty, then prepare your lysis buffer solution.
- Weigh each cell pellet using the balance and add 1 mL of lysis buffer per g of cell pellet.
- Note: first, you'll need to weigh a 50 mL conical tube.
- Resuspend each pellet completely in the lysis buffer then transfer the cell suspension to a 2 mL eppendorf tube.
- Note: You will need to cut the tip off of a P1000 pipette tip to transfer cell suspension.
- Add lysozyme (stock concentration of 50 mg/mL) to each cell suspension such that the final concentration is 300 μg/mL.
- Incubate in the 4 °C cooler for 1 h on the nutator.
| Stock reagent | Final concentration of stock reagent in lysis buffer | Volume of stock reagent |
|---|---|---|
| 1 M Tris (pH = 7) | 50 mM | |
| 1 M NaCl | 150 mM | |
| 40% glycerol | 10% | |
| 1 M DTT | 1 mM | |
| 100 mM AEBSF | 1 mM | |
| H2O | add for a total of 3 mL of lysis buffer |
Part 2: Prepare Ni-NTA affinity column
- Obtain a 250 μL aliquot of 50% slurry from the front laboratory bench.
- Add 1 mL of 1X PBS and mix.
- Centrifuge for 1 min at 3300 rpm.
- Carefully aspirate only the liquid.
- Add another 1 mL of 1X PBS and mix.
- Centrifuge for 1 min at 3300 rpm.
- Leave the tube on your benchtop until you are ready to add the cell lysate in Part 3.
- Immediately before you add your cell lysate, carefully aspirate the liquid.
Part 3: Purify FKBP12 protein
- Retrieve your lysed cell pellets from the 4 °C cooler.
- Transfer 15 μL from each tube (-ITPG and +IPTG) into labeled 1.5 mL eppendorf tubes and give the aliquots to the teaching faculty.
- You will use these aliquots during the next laboratory session to examine protein yield using polyacrylamide gel electrophoresis (PAGE).
- Calculate the volume of 1 M MgCl2 to add for a final concentration of 10 mM in your remaining cell lysate.
- Add the volume of MgCl2 you calculated in the previous step and 10 μL of DNase to the tube that contains the IPTG-induced sample.
- You will only complete the purification protocol for the IPTG-induced sample!
- Incubate for 30 min in the 4 °C cooler.
- Centrifuge your sample for 30 min at 16,000 rcf in the 4 °C cold room.
- Alert the teaching faculty when you are ready to centrifuge your sample and you will be escorted to the cold room.
- Check that the supernatant in your sample is clear with little to no 'cloudiness'.
- Carefully use your P1000 pipet to remove the PBS supernatent from the column you prepared in Part 2 and dispose in your waste container.
- Load the supernatant from your protein solution onto the column.
- Incubate the column with the protein solution at 4 °C for 30 min to promote binding.
- Retrieve your tube and centrifuge at 3300 rpm for 1 min, then use a pipet to remove the supernatent and dispose in your waste container.
- Obtain an aliquot of PBS buffer containing 10 mM imidazole from the front laboratory bench.
- Wash the column by adding 1.5 mL of the PBS buffer containing 10 mM imidazole to the column and invert, then centrifuge at 3300 rpm for 1 min.
- Transfer the supernatant to a fresh, well-labeled 2 mL eppendorf tube.
- Repeat Step #13 a total of three times.
- Elute your FKBP12 protein by adding 1 mL of PBS buffer containing 250 mM imidazole to the column and invert, then centrifuge at 3300 rpm for 1 min.
- Transfer the purified protein sample (the supernatant) to a fresh, well-labeled 15 mL conical tube.
- Repeat Step #15 a total of three times, adding the supernatant to the same conical tube.
- Submerge your dialysis cassette in cold PBS for 2 minutes.
- Carefully use a P1000 pipetman to transfer your eluted purified protein to a dialysis cartridge by removing cap and injecting the solution between the membranes.
- Give your wash samples from Step #10 and your used purification column to the teaching faculty.
Your purified protein solution will dialyze for ~18 hr in PBS buffer to remove the imidazole as it may interfere with the small molecule screen. Following dialysis, the teaching faculty will transfer your purified protein solution to a fresh eppendorf tube and store it at 4 °C until your next laboratory session.
Reagents
- PBS: phosphate saline buffer (VWR # 97064-158)
- Ni-NTA column (Qiagen)
- AEBSF: 4-(2-aminoethyl)benzenesulfonyl fluoride (Sigma # A8456-25MG)
- Lysozyme from chicken egg white (Sigma # L4919-500MG)
- DNase I (Sigma # AMPD1-1KT)
- DTT : DL-dithiothreitol (Sigma # D9779-250MG)
- imidazole (Sigma # I2399-100G)
Next day: Evaluation of purified protein
Previous day: In silico cloning and induction of protein expression