20.109(S15):Flow cytometry and paper discussion (Day6)
Contents
Introduction
We hope that you’ll leave lab today with a sense of accomplishment, after inspecting your raw flow cytometry data and then calculating NHEJ repair values. Unfortunately or excitingly – depending on your perspective – it turns out in scientific research that the hard work is just beginning once the data is quantified! Interpreting the data and drawing (sometimes tentative) conclusions requires deep reading and thinking – a process that shouldn’t be rushed. To help get you started, let’s review a few key elements of DNA repair and of the NHEJ pathway in particular.
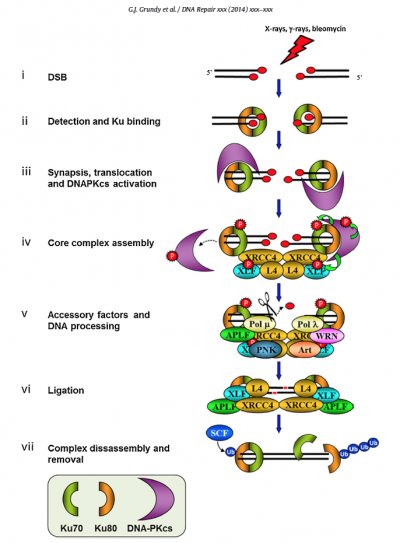
Our discussions of NHEJ have primarily been focused on Ku80 and DNA-PKcs, along with a few other key players such as Ligase IV, and will remain so here. However, the schematic below from the review by Grundy et al. highlights that many accessory molecules have their own roles to play. Recall that Ku80, with its dimer partner Ku70, completes the first step of NHEJ repair by binding to DNA double-strand breaks (DSBs). Keep this function in mind as you consider how a Ku80 knockout strain might respond to DNA damage. The Ku 80/70 dimer quickly recruits DNA-PKcs, the catalytic subunit of DNA-dependent protein kinase, to form the DNA-PK complex. Although DNA-PKcs has some binding affinity for DSBs, this affinity is increased by two orders of magnitude by the presence of the Ku dimer, and the kinase activity itself cannot proceed in the absence of Ku. Interestingly, although the kinase activity of DNA-PK is known to be important, it is not certain precisely which phosphorylation events are absolutely required for NHEJ.
The one piece of wet lab work that you will do next time is complete your NHEJ inhibitor validation assay. Clonogenic assays of mammalian cells have over a 50 year history, as mentioned in the methods paper by Franken et al. They are useful for assessing the reproductive capacity of cells after irradiation and other types of damage. We will diverge somewhat from the Nature Protocols paper, but it is useful for introducing terms such as the plating efficiency and the surviving fraction. Specifically, we do not need to fix our cells in an independent step, because the stain that we will use contains methanol. (Correction! Our stain contains very little methanol, so fixing does not appear crucial for short-term staining.) Second, we will not use the crystal violet stain, which binds DNA, but instead a Coomassie derivative, which targets proteins. In fact, you may recognize Coomassie as the go-to stain for SDS-PAGE. Protein binding by the dye occurs primarily via arginine, as well as other basic and aromatic residues, as described here. We will use a variant of the original Coomassie Brilliant Blue stain called BioSafe Coomassie.
Most of your time today will be spent at the computer, quantifying flow cytometry data. Recall from the M2D4 introduction that we will proceed in three main steps.
First, reporter expression for GFP and BFP alike will be calculated by multiplying percentage of positive cells by fluorescence intensity (FI). We have a choice of whether to use mean, geometric mean, or median fluorescence intensity. Median fluorescence is least susceptible to being influenced by a few outliers, while geometric mean is generally more appropriate for log scale data than arithmetic mean. For normally distributed populations, all three values should be pretty similar. In practice, we have found that while mean and median FI are very different values, after normalization the ultimate NHEJ repair values are quite similar, so we will use the mean value.
The second step is to calculate the ratio of BFP to GFP reporter expression for each sample. The final step is divide the damaged-BFP:GFP ratio by the maximal possible “repair,” namely the intact-BFP:GFP ratio. Convince yourself that this parameter essentially provides the fraction of BFP plasmids repaired.
Protocols
Part 1: Paper discussion
As described in the Day 5 homework, we will be discussing the Goglia et al. paper in class today.
Technical Background
The paper by Goglia et al. utilizes a fluorescence based DNA repair sensor similar to the one that you are employing in Module 2. There are, however, some important differences in the construction and function of the EJ-RFP (end joining-red fluorescent protein) sensor versus the pMAX-BFP-MCS sensor that was constructed for 20.109. Another paper from the same lab, published in 2013, details the development of the EJ-RFP sensor. You do not need to read the entire paper, but make sure that you understand how the sensor works so that you can fully grasp the high throughput screen that was completed in the Goglia et al. manuscript.
In particular, please read the Introduction and the first two Results sections of the 2013 paper. You will find this background presentation to be helpful for understanding the DNA repair sensors in the Goglia et al. paper.
Discussion Topics
Content
The following questions will guide our in-class discussion; consider them as a starting point rather than a check-list.
(A) DNA repair background
- What is the difference between canonical NHEJ and (what the authors term) mutagenic NHEJ? What type of NHEJ does your sensor measure?
- How did the authors develop a specific screening tool for mNHEJ versus cNHEJ?
(B) Drug screening background
- What is the difference between a "reverse chemical genetic" screen and a "forward chemical genetic" screen? Why would you use one versus the other?
- What type of equipment did the authors use to perform the drug screen? Think about a couple reasons why this would be not only convenient, but also important for the study.
- Why was DMSO added at 1% in wells not containing drug?
- What was the purpose of adding Shield1 and TA? The authors state that the "absence" of these ligands is a positive control. What do they mean?
- What is an orthogonal assay? What is the purpose of performing these types of assays?
- What orthogonal assays were performed to confirm the original hits?
Figures & Results
We will not discuss all of the figures in this paper. Concentrate on the Results sub-sections and Figures outlined below. Be prepared to discuss all of the figures listed below. It is completely fine to have questions about the paper and to not fully grasp all of the material, but it is expected that you will have put forth a good faith effort to do so.
- Figure 1
- Figures 1A and 1B are typical schematic diagrams that you find within journal papers. Why are these helpful and what specific information do you obtain from these sub-panels?
- Figure 1C provides an example of the type of output data obtained. What factors do the authors report are important to achieve adequate cell segmentation?
- The remainder of Figure 1 shows important control experiments. Answer the following questions while you read:
- What controls are shown and why is it important to show these controls in a figure?
- What is a Z'-factor and why do the authors use it?
- Do you feel convinced that the data obtained in the large inhibitor screen will be believable after reading about and examining Figure 1?
- Figure 2
- What is a RADaR plot and how do you read it? (Part of selling your science is coming up with memorable acronyms.)
- Explain the significance of Figures 2B and 2C.
- Why did the authors start with the LOPAC 1280 library (and what is it)?
- Figure 3
- This figure shows a graphical representation of the 20,000 compound screen. How many times was this replicated? Where did the 20,000 compound library come from?
- Figure 4
- This is another great example of an useful schematic diagram in this paper. Consider all of the information that is contained in this small flowchart and, as you read, keep track of how many times you reference it.
- As you put together your Mod2 report, think about how you might use schematic diagrams to help the reader understand your study.
- Figure 5
- What is RU-0084411 and why is it an interesting hit in the screen? Why might the authors (and pharmaceutical companies) be especially interested in following up on this type of hit?
- The curves shown under Figure 5A-2&3 are generally referred to as 'dose-response' or 'inhibition' curves. Many dose-response curves have a sigmoidal shape. How does one estimate the IC50 from analyzing this type of data?
- Do any of the plots shown in Figure 5A give you pause with respect to future use of Mibrefradil as a clinical NHEJ inhibitor?
- Figure 6
- What is an orthogonal assay in the context of this paper? Why is it important to do them?
Discussion & Conclusions
Please read the entirety of the Discussion section.
- List three reasons why the authors state that their study is novel. What type of evidence do they use to convince you?
- List a couple limitations of the paper (that the authors address in this section).
The purpose of a Discussion section is (at least) four-fold:
- Provide a summary and explanation of the data in the paper. This is the place to do all of your interpretation. For example, in the third paragraph of the Discussion section that starts "We identified several novel molecules...", the authors admit to being surprised at some of their findings. They then postulate why these findings might be real and suggest further studies that would be required to further tease apart the current data.
- Convince the audience that your study makes a contribution to the field. The Discussion section is the place to compare and contrast your current results with those that have already been published. Why are your results interesting and important? Re-visit your Introduction -- what was your 'big picture' motivation? How did your study impact that?
- Admit your limitations. No study is perfect, don't let anyone tell you that it is! Perhaps your data doesn't quite get to the answer and there is a technical limitation -- tell the audience. Perhaps the cell system you are using isn't the optimal one (which may or may not be available) -- tell the audience. Perhaps the data from your DNA repair assay is noisy and you know why -- tell the audience. The Discussion section should admit to limitations and suggest specific ways to address them.
- Suggest the next big thing. Where does your study leave off? Since you are now the expert -- what is the next most important thing to do?!?
Part 2: Flow cytometry analysis
Overview:
- You will begin by looking at images from the instructor samples to learn how to read the flow cytometry plots and summary statistics.
- Next you will peek at your own images and form preliminary expectations about your data set.
- Finally, you will work in Excel to precisely calculate the NHEJ repair value for each of your three conditions (two replicates each).
Protocol:
- The pdf files with your data are posted on the M2D6 Talk page.
- The instructor samples are listed in the table below. From this table, and from the T/R and W/F image sets, try to address the questions below.
- Background. The scatter data is used – in three steps – to make gate P3, which should consist primarily single cells. Next a gate that we called Live cells was set based upon the addition ToPro3 to the media. ToPro3 can only pass through the membranes of dead cells, staining them with a dye that fluoresces when exited with 647 nm wavelength light. The cytometer we used calls this channel the APC channel (because the dye APC also fluoresces at that wavelength). So, using the plot that shows FSC vs APC we can gate-in the live cells (exclude the dead ToPro3+ cells).
- From the cells gated in Live Cells, two sub-gates are made that capture all GFP-positive cells ("Green cells" gate) and all BFP-positive cells ("Blue cells" gate). Both singly and doubly positive cells are included in each gate. It is important to read the "% Parent" statistics: these indicate XFP-positive cells as a percentage of all the cells in Live Cells. The "% Total" statistics include debris, aggregates, and clearly dead cells!
- What percent Green cells are in the mock sample on each day? What about Blue cells?
- What percent of singly-transfected cells express GFP? Do cross-day replicates agree well or not?
- What percent of singly-transfected cells express BFP? Do cross-day replicates agree well or not?
- Now, start to look at your K1-intact conditions (and possibly those of your classmates).
- What percent of co-transfected cells express GFP? Express BFP? How many express both?
- How is within-day and cross-day replicate agreement for the co-transfected samples?
- Answer the following by looking at a team that used DMNB (hint: there is a spreadsheet on the Talk page that will help you locate those samples).
- Does DMSO appear to affect scatter profiles? What about affecting co-expression?
- Hint: you'll need to compare the K1 intact samples from a DMNB group versus a different group.
- After you understand the instructor data, skim over your 12 sample plots. Can you see apparent differences between K1, K1+inhibitor, and xrs6?
- Now that you have a good conceptual understanding of the data, it's time to crunch some numbers. Open the .csv file and save it as a newly named .xlsx file.
- Begin by deleting all of the rows except the twelve containing your own dataset.
- Next delete all of the columns except the few that interest you. Keep in mind that you need to know Green cell and Blue cell gating as a % of the parent gate, Live Cells. Class-wide, you are only required to do your calculations based on mean fluorescence intensity (MFI), but you should also keep the median data in case others want to use it.
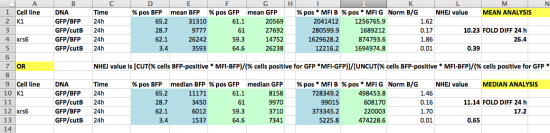
- We recommend that you prepare a new Excel file with your NHEJ equations, and just copy-paste in the appropriate % and MFI data; this approach is a versatile one. Your final worksheet might look similar to the screenshot below.
- Remember that for each of the twelve wells you should calculate raw reporter expressions and a BFP/GFP normalized value. Then, for each intact/cut pair you can calculate an NHEJ value. In this way, we should have quadruplicate NHEJ values for most repair topology/cell population conditions, which will allow us to do statistical comparisons.
Reference information:
| Day | Tube # | Condition |
|---|---|---|
| T/R | 1 | Mock transfection |
| T/R | 2 | GFP Intact Only |
| T/R | 3 | BFP Intact Only |
| W/F | 1 | Mock transfection |
| W/F | 2 | GFP Intact Only |
| W/F | 3 | BFP Intact Only |
You must email your Excel sheet to Shannon (T/R) or Leslie (W/F) before leaving lab today. We instructors will post a summary file for ease of class-wide data analysis by Wednesday evening or Thursday morning.
Previous Day: DNA repair assays
Next Day: Data analysis