20.109(F16):Complete immuno-fluorescence assay (Day6)
Contents
Introduction
The ability to bind specific proteins using antibodies, or immunoglobulins, is critical in immuno-fluorescence labeling and Western blot analysis. Antibodies are typically 'raised' in mammalian hosts. Most commonly mice, rabbits, and goats are used, but antibodies can also be raised in sheep, chickens, rats, and even humans. The protein used to raise an antibody is called the antigen and the portion of the antigen that is recognized by an antibody is called the epitope. Some antibodies are monoclonal, or more appropriately “monospecific,” and recognize one epitope, while other antibodies, called polyclonal antibodies, are in fact antibody pools that recognize multiple epitopes. Antibodies can be raised not only to detect specific amino acid sequences, but also post-translational modifications and/or secondary structure. Therefore, antibodies can be used to distinguish between modified (for example, phosphorylated or glycoslyated proteins) and unmodified protein.
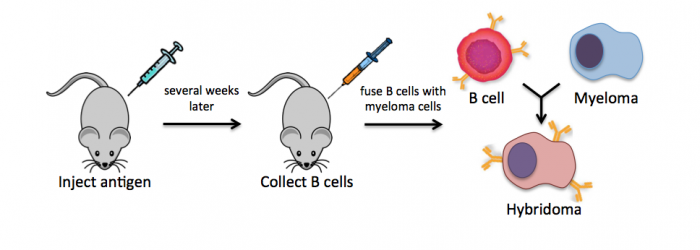
Monoclonal antibodies overcome many limitations of polyclonal pools in that they are specific to a particular epitope and can be produced in unlimited quantities. However, more time is required to establish these antibody-producing cells, called hybridomas, and it is a more expensive endeavor. In this process, normal antibody-producing B cells are fused with immortalized B cells, derived from myelomas, by chemical treatment with a limited efficiency. To select only heterogeneously fused cells, the cultures are maintained in medium in which myeloma cells alone cannot survive (often HAT medium). Normal B cells will naturally die over time with no intervention, so ultimately only the fused cells, called hybridomas, remain. A fused cell with two nuclei can be resolved into a stable cell line after mitosis.
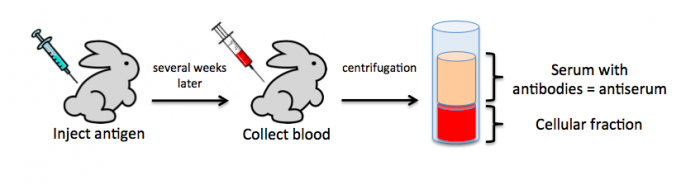
To raise polyclonal antibodies, the antigen of interest is first purified and then injected into an animal. To elicit and enhance the animal’s immunogenic response, the antigen is often injected multiple times over several weeks in the presence of an immune-boosting compound called adjuvant. After some time, usually 4 to 8 weeks, samples of the animal’s blood are collected and the cellular fraction is removed by centrifugation. What is left, called the serum, can then be tested in the lab for the presence of specific antibodies. Even the very best antisera have no more than 10% of their antibodies directed against a particular antigen. The quality of any antiserum is judged by the purity (that it has few other antibodies), the specificity (that it recognizes the antigen and not other spurious proteins) and the concentration (sometimes called titer). Animals with strong responses to an antigen can be boosted with the antigen and then bled many times, so large volumes of antisera can be produced. However animals have limited life-spans and even the largest volumes of antiserum will eventually run out, requiring a new animal. The purity, specificity and titer of the new antiserum will likely differ from those of the first batch. High titer antisera against bacterial and viral proteins can be particularly precious since these antibodies are difficult to raise; most animals have seen these immunogens before and therefore don’t mount a major immune response when immunized. Antibodies against toxic proteins are also challenging to produce if they make the animals sick.
In your experiment, you will use a primary antibody to bind the γH2AX foci. Then a secondary antibody will be used that is specific to the conserved region of the primary antibody. The use of secondary antibodies allows researchers to tag the primary antibody. In our assay, the tag is a 488 nm fluorescent dye that will enable us to visualize double-strand breaks via microscopy. As a reminder, during the last laboratory session you seeded cells for the H2AX assay. Since then, the teaching faculty treated the appropriate wells with H2O2 and fixed all the wells with paraformaldehyde. Your goal for today is to permeabilize the cells, which will enable the antibodies to enter the cells and bind γH2AX.
Protocols
Part 1: BE Communication Lab workshop
Our communication instructors, Dr. Sean Clarke and Dr. Diana Chien, will join us today for a workshop on writing an informative and concise abstract.
Part 2: Complete H2AX assay
At this point in the assay sterility is no longer a concern and you will complete the following steps in the main laboratory at your bench.
- Obtain your 12-well plate from the 4 °C cooler.
- Gather aliquots of Triton X-100 and TBS from the front laboratory bench.
- Prepare 5 mL of a 0.2% Triton X-100 solution (v/v) using the TBS.
- Aspirate the 1x PBS from the wells in your 12-well plates.
- Permeabilize the cells by adding 500 μL of the Triton X-100 solution to each well and incubate for 10 min at room temperature.
- Obtain an aliquot of blocking solution from the front laboratory bench.
- Aspirate the Triton X-100 solution and add 500 μL of blocking solution to each well, then incubate for 60 min at room temperature.
- With 15 min remaining of the blocking solution incubation, prepare the primary antibody and staining chamber.
- Dilute the mouse anti-γH2AX antibody 1:100 in a 700 μL aliquot of fresh blocking solution.
- Obtain a staining chamber from the front bench. Cut a piece of parafilm to fit inside, adhere parafilm to bottom of chamber and add a damp paper towel to each side of the parafilm. Label parafilm with experimental details.
- Obtain a fine gauge (26 3/8) needle and a pair of tweezers from the front laboratory bench.
- Carefully press the tip of the needle against the benchtop to bend it into a right angle such that the beveled side of the needle is the interior angle.
- Use the 'hook' created in Step #8 to lift the coverslip from the bottom of the well, then use the tweezers to 'catch' the coverslip.
- In total, you only need to visualize one coverslips from each experimental condition; therefore you have one well that can be used as practice.
- When you are confident with your ability to retrieve the coverslips from the wells, move a total of 4 coverslips from the 12-well plate to the staining chamber. Cell-side UP!
- The cell-side of the coverslip is the side that was facing up in the well of the 12-well plate.
- Quickly add 150 μL of the diluted primary antibody solution to each coverslip before moving the next. Do not let the coverslips dry.
- Carefully move your staining chambers to the 4 °C cooler.
Your samples will incubate at 4 °C in the primary antibody solution for ~48 h. The teaching faculty will replace the primary antibody solution with the secondary antibody solution, Alexa Fluor 488 goat anti-mouse diluted 1:200 in blocking solution, 1 h prior to the next laboratory session.
Part 3: Statistics practice
Review these data from an experiment where cells were exposed to increasing amounts of radiation. Your goal is to determine if a statistically significant amount of DNA damage was induced. For the purpose of this exercise, the values in the spreadsheet are in arbitrary units of 'DNA damage', where the higher numbers indicate more damage.
Think back to the lecture delivered by Dr. Lyell on statistical analysis – how will you convince someone that the DNA damage was significant? You may find this spreadsheet, originally created by Prof. Bevin Engelward and modified by the 20.109 staff, helpful for this exercise. At a minimum, you should post a bar plot of the data with 95% confidence intervals and indicate if there is a statistically significant difference (i.e. provide a p-value) between conditions in your Benchling notebook.
Part 4: Data analysis for CometChip assay
With the remaining time, continue to process your CometChip assay data using the protocol described on M1D5.
Reagents list
- paraformaldehyde 4% (VWR)
- permeabilization buffer: 0.2% Triton in Tris buffer saline (TBS, Invitrogen)
- blocking buffer: 1% bovine serum albumin (BSA) in TBS
- primary antibody to γH2AX, mouse (Millipore)
- goat anti-mouse secondary antibody (Invitrogen)
Next day: Analysis of sub-nuclear foci
Previous day: Develop approach for sub-nuclear visualization of DNA damage