20.109(F15):Growth of phage materials (Day1)
Contents
Introduction
The accomplishments of the natural world can inspire us to great engineering feats. Biomineralization is one particularly impressive trick accomplished in nature. Vertebrates, invertebrates and plants all are able to precisely position inorganic substrates into crystalline order. For example, calcium carbonate forms unstructured dust in the absence of genetically-programmed organizers, but the same material can be made into the hard and luminous shells of sea creatures as in the case of the abalone shell. Similarly, diatoms organize silicon dioxide into intricate patterns that manufacturers of electronic components are unable to recreate. In one more instance, bacteria align iron inside their cytoplasm to form magnetic rods on the submicron scale. These feats are accomplished without harsh chemicals, without extreme temperatures, and without noxious wastes that poison the nests of the organisms themselves. Humans have much to learn from nature’s successes. In the upcoming weeks we’ll use a virus that infects bacteria, namely the bacteriophage M13, and we'll rely on the self-assembling coat of this virus to biotemplate iron for the construction of a battery electrode. The interaction of metals with a protein on the phage coat yields nanoscale-particles with useful energetic properties, as we’ll see.
About M13
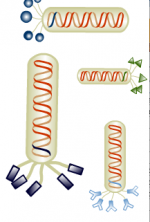
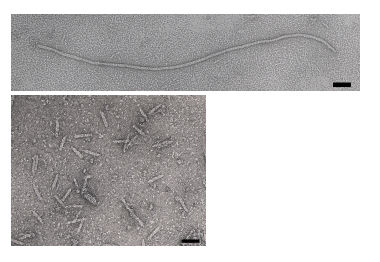
The bacteriophage M13 is a member of the filamentous phage family. It has a long (~900 nm), narrow (~6 nm) protein coat that encases a small (~6.4 kb) single stranded DNA genome. The genome encodes 11 proteins, five of which are exposed on the phage’s protein coat and six of which are involved in phage maturation inside its E. coli host. The phage coat is primarily assembled from a 50 amino acid protein called pVIII (or p8), which is sensibly enough encoded by gene VIII (or g8) in the phage genome. For a wild-type M13 phage, it takes ~2700 copies of p8 to make the ~900 nm long coat. The coat's dimensions are flexible though and the number of p8 copies adjusts to accommodate the size of the single stranded genome it packages. For example, when the phage genome was mutated to reduce its number of DNA bases (from 6.4 kb to 221 bp) [2], then the p8 coat “shrink wrapped" around the reduced genome, decreasing the number of p8 copies to less than 100. Electron micrographs of the resulting “microphage” and its wild type parent are shown below (image courtesy of Esther Bullitt, Boston University School of Medicine), where the black scale bar in each image is 50 nm long. And what about the upper limit to the length of the phage particle? Anecdotally, viable phage seems to top out at approximately twice the natural DNA content. However, deletion of a phage protein (p3) prevents full escape from the host E. coli, and phage that are 10-20X the normal length with several copies of the phage genome can be seen shedding from the E. coli host.
Phage life-cycle
The general stages to a viral life cycle are: infection, replication of the viral genome, assembly of new viral particles and then release of the progeny particles from the host. Filamentous phages use a protein at their tip, namely p3, to contact a bacterial structure known as the F pilus to infect E. coli. The phage genome is then transferred through the pilus to the cytoplasm of the bacterial cell where resident proteins convert the single stranded DNA genome to a double stranded replicative form (“RF”). This DNA then serves as a template for expression of the phage genes and produces new phage particles that shed off the surface of the infected cell. Other phage are known to lyse their host cells but in the case of M13 and E. coli, they co-exist, allowing the growth of both host and virus, though the infection does slow down the doubling time of the E. coli.
Phage display
Phage display has been used for decades as a tool for discovery. This technique exploits natural selection and identifies functional peptide sequences that can be fused to the phage coat. Most often the p3 protein at the phage tip is used for phage display because, despite the limited number of displayed peptides per phage (on the order of 5), there is enough flexibility to accommodate peptides of 20 to 30 amino acids. The other protein used for phage display, p8, is present at a much higher copy number per phage (on the order of 2700) but it has limited flexibilty. The semi-crystalline packing of p8 on the phage coat restricts fusions to only 4 to 6 neutral or negatively charged amino acids. For scientists who can tolerate a mix of p8 proteins on the phage coat for their work, there are phage-display variations that mix and match fusion and wild-type proteins on a phage coat, but for those who want phage of a particular form, the options are limited.
Nonetheless, peptides with remarkably diverse functions have been isolated with phage display. Once the fusion site is chosen, a library of sequences encoding random peptides can be synthesized and cloned. In this way a pool of phages, each with different fusions, can be made. Finally, the phage pool can be screened for interesting behaviors or properties. Peptide-fusion proteins to p8 or p3 that include stop codons or intolerable sequences are largely lost from the population after the first round of “panning.” Other phages that possess the desired trait remain and can be directly isolated from the pool or further enriched by a second, third, fourth round of panning. Ultimately anywhere from 10 to 1000 candidate sequences may remain from a starting pool of 1 billion.
Despite phage display techniques being available for more than a generation, this tool has been applied only recently to the search for novel materials. Largely it’s been Prof. Angela Belcher and her lab who highlighted and then demonstrated the usefulness of this search tool for finding peptides that interact with materials to meet human needs. That M13 could interact with inorganic materials could not have been predicted from the original genetic studies on the phage, but there was also no one who had tried it! Phage that can bind to cobalt oxide, gold, iridium and indium tin oxide are all in-hand thanks to their work (e.g see reference [3]). Today you will harvest and titer a phage that can bind to iron which you will use to build nanowires next time.
Protocols
In advance of this lab, a bacterial host E. coli XL1-blue was infected with a modified M13 phage with a mutated p8 amino acid sequence that is able to bind Fe. The p8 modification (=DSPHTELP) was isolated from a library of p8 mutants that enable the phage to bind single wall carbon nanotubes (SWCNT). Today you will harvest the phage from the supernatant of the infected bacterial culture and setup the first step of the Fe(III)-phage biomineralization reaction.
Part 1: Phage purification
- Divide the overnight culture (~100 mL volume) into two 50 mL conical tubes.
- Label with your group information using a marker (not the small dots or tape; these can come off in the rotor).
- Spin at 3,250 rpm for 15 minutes using the centrifuge located on the prep bench. The teaching faculty will assist you in putting your samples into the rotor.
- Transfer the supernatant into two fresh 50 mL conical tubes.
- This should be done with a plastic pipet so you are able to measure the volume of supernatent.
- Add 1/4th the volume of supernatent you measured in Step #4 of 20% PEG-8000/2.5 M NaCl solution to your 50 mL conicals containing your phage samples.
- Invert to mix then incubate on ice 60 minutes.
- Feel free to prepare your (NH4)2Fe(SO4)2 solution per Part 3, Step #2.
- Spin at 3,250 rpm for 20 minutes. A white pellet may be visible... these are your precipitated phage. If you can't see a pellet keep going, but mark where the pellet you can't see is in the tube and don't scrape a tip against it or you will accidentally remove it.
- Remove the supernatant by pouring most of it down the sink and the rest with aspiration (carefully so as not to disturb the pellet).
- Resuspend the pellet in 3 mL sterile water. This is best done by adding 3 mL of H2O to one of the conical tubes, washing the water up and down the side of the tube with the phage pellet, and then transferring the 3 mL of phage solution to the second tube and dissolving that pellet by washing the water up and down the side of the tube.
- Split the phage solution between three eppendorf tubes.
- This should be done with a pipet so you are able to measure volume of phage solution.
- Add 1/4th the volume of phage solution you measured in Step #9 of 20% PEG-8000/2.5M NaCl solution to your eppendorf tubes.
- Invert to mix. Then incubate on ice for 15 minutes.
- Centrifuge the tubes full speed in a microfuge for 10 minutes.
- Aspirate the supernatant and resuspend the pellets (if you can see them) in 1.5 mL diH2O - using 0.5 mL to resuspend each pellet and then pooling the volumes into a larger (2 mL) microfuge tube. This is your phage stock.
- If the solution looks at all cloudy, spin in a room temperature microfuge for 1 minute more and move supernatant with the phage to a new tube.
Part 2: Measuring concentration of phage
With this technique you will calculate the concentration of phage in your stock using the spectrophotometer. This method can approximate the number of phage based on the ability of the virions to absorb ultraviolet light. The number of phage is calculated by the formula:where
- the molar extinction coefficient of the phage and the average size of a DNA base are collected into the constant
- the absorbance at 269 nm reflects the protein and DNA content in the solution
- the absorbance at 320 nm corrects for the naturally high baseline value of the solution
- the number of DNA bases in the M13 phage genome is ~7220.
This method for titering the phage stock is less informative than the traditional plaque method (known as titering) since materials other than phage might be contributing to the absorbance readings. Thus, the number of infectious particles isn't truly known. Since infectivity is not critical for the synthesis of Fe(III)-phage nanowires, however, we will be using spectrophotometry only.
- Dilute the phage stock you have 1:10 by adding 70 μL of the phage to 630 μL of diH20, vortex to mix and then move this solution to a quartz (not plastic!) cuvette.
- A few things to be aware of when using quartz cuvettes:
- They are very expensive.
- The lab has very few.
- When you are done using your cuvette, you should carefully clean it by shaking out the contents into the sink and rinsing it once with 70% EtOH, then two times with water. Quartz cuvettes get most of their chips and cracks when shaking out the contents since it is so easy for the cuvette to slip from wet fingers or be hit against the sink. Don’t let this happen to you.
- A few things to be aware of when using quartz cuvettes:
- Read the absorbances of your phage dilution at 269 and 320 nm, using diH20 in a second quartz cuvette to blank the spectrophotometer at each wavelength.
- Calculate the number of phage particles/mL using the formula shown above.
Part 3: Begin Fe(III)-phage biomineralization
In the first step of the biomineralization process, (NH4)2Fe(SO4)2 is added to the purified phage. The iron will complex with the negatively charged p8 protein on the coat of the phage particles. You will construct your cathode using the active material generated from this biomineralization reaction.
- Using the number of phage you calculated in Part 2, determine how many additional phage you need to have a total phage count of 2 x 1011, 2 x 1012, or 2 x 1013. Note: this is a total quantity and not a concentration.
- Think about which amount you would like to use in your biomineralization reaction and use the Discussion page to reserve this value. Only two groups can sign up for each quantity.
- The extra phage stock is on the front bench and is at the following concentration: 2.4 x 1013 pfu/mL. Calculate the volume of this stock you need to add to have the appropriate number of phage in your reaction.
- Confirm your math with the teaching faculty then add the appropriate volume of the phage stock to your purified phage sample.
- In a clean flask, prepare 100 mL of 10 mM (NH4)2Fe(SO4)2 (MW = 392.14 g/mol). To mix the solution, add a stirbar and use the stirplates near the weigh balance.
- Add your phage sample from above to the (NH4)2Fe(SO4)2 solution.
- To ensure you add all of the phage in your sample, use a P1000 pipet to transfer your phage to the (NH4)2Fe(SO4)2 solution.
- Wash any phage that may be clinging to the side of your tube by using your P1000 to transfer 1 mL of of the (NH4)2Fe(SO4)2 solution from your flask to your now empty tube and vortex.
- Again, with your P1000 transfer the (NH4)2Fe(SO4)2 solution with any residual phage from your tube to your flask.
- Cover your flask with parafilm and put your reaction in the 4°C cooler on the designated shelf.
- Be sure to clearly label your flask!
Because the initial step of the biomineralization process is time-sensitive, George will quench the reaction in ~12 h by adding 100 mL of 1 mM Na:PO4. In this step, the phosphate precipitates the Fe(III) onto the p8 proteins of the M13 phage coat to generate nanowires. You will see a demo of this reaction in the next laboratory class.
Reagents list
20% PEG 8000, 2.5 M NaCl
LB
- 10 g tryptone
- 5 g yeast extract
- 10 g NaCl
- 1 L deionized water
- Autoclaved 30 minutes with stirbar.
TBS
- 50 mM Tris
- 150 mM NaCl
- pH 7.6
Next day: Purify active materials