Protocols for cell culture
Introduction
This experiment seeks to better understand the effect of CytoD on cells. Since there are significant rheological changes of 3T3 fibroblast with CytoD, it is reasonable to assume that this chemical may modify the fibroblast cytoskeleton. The most important component of the mammalian cell cytoskeleton is actin, so we will image actin structures in these cells with and without CytoD treatment.
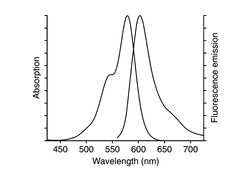
The actin cytoskeleton is visualized using the toxin phalloidin labeled with Alexa Fluor 568. The excitation maximum is at 578nm and emission maximum is at 600nm. Phalloidin is a fungal toxin (small organic molecule) that binds only to polymerized filamentous actin (F-actin), but not to actin monomers, G-actin. (It is a toxin because it hinders actin disassembly).
Cell Fixation and Labeling Protocol
Prepare a dish of fixed fibroblast cells (NIH 3T3) with actin labeled with phalloidin-Alexa Fluor 568. The labeling protocol is as follows: The starting point is as before - cells cultured in dishes containing DMEM++, at approximately 60% confluency. This is about the optimum percentage of cell population. If cells are too crowded, they will not stretch properly and show their beautiful actin filaments. Note also that these cells remain alive until the addition of formaldehyde, therefore requiring that any buffer/media added to be pre-warmed.
- Pre-warm 3.7% formaldehyde solution, DMEM and PBS in a 37°C water bath. Keep the solution wrapped in foil to protect from light.
- Retrieve an aloquat of CytoD from the freezer and warm in the water bath to 37°C.
By a very similar procedure, you should also prepare cells treated with CytoD: Before adding the formaldehyde, add 1 mL of the pre-warmed 10 μM CytoD solution and incubate at 37°C for 20 min. Afterwards, wash with DMEM++ twice.
- After you get your dishes from the TA or Instructor, first remove the medium with a pipette and wash each dish twice with 2 mL of pre-warmed phosphate buffered saline (PBS) at pH 7.4 (also in the water batch). Pipet into the dish gently to avoid washing away cells.
- Carefully pipet 400μL of 3.7% formaldehyde solution onto the central glass region of the dish and leave it for 10 minutes at room temperature. This "fixes" the cells, i.e. cross-links the intracellular proteins and freezes the cell structure.
- Wash the cells three times with 2 mL PBS (no longer needs to be pre-warmed as the cells are dead).
- Extract each dish with 2 mL 0.1% Triton X-100 (a type of soap solution in PBS) for 3-5 minutes. (Extraction refers to partially dissolving the plasma membrane of the cell.)
- Wash the cells 2-3 times with PBS.
- Incubate the fixed cells with 2 mL 1% BSA (bovine serum albumin) in PBS for 20 minutes. (BSA blocks the nonspecific binding sites.)
- Wash cells twice with plain PBS.
- To each cell dish, add 200 μL of Alexa Fluor 568 phalloidin solution (specific binding to F-actin) pre-mixed in methanol and PBS. Carefully pipet this just onto the center of the dish, cover with aluminum foil, and incubate for 45 min. at room temperature.
- Wash three times with PBS.
- You can now store the sample at +4°C (normal refrigerator) in PBS for a few days, wrapped in parafilm and foil. It can also be stored in mounting medium for up to 1 year.
Actin Imaging
Since actin filaments and stress fibers are nm-scale objects, they are much dimmer than fluorescent beads or the dye solution - care must be taken to get good images of the cytoskeleton. You may need to cover the scope to reduce room light contamination.
Adjust the gain and exposure of the camera to get the best picture. Be sure to keep the same exposure conditions, however, for both untreated and treated cells.
Using the 40x objective, take some good images each of treated and untreated cells.