Difference between revisions of "Lab Manual: Optical Microscopy"
MAXINE JONAS (Talk | contribs) m (moved Lab Manual:Optical Microscopy to Lab Manual: Optical Microscopy: Added space for consistency with other links on the wiki) |
MAXINE JONAS (Talk | contribs) (→Lab manual pages) |
||
| Line 117: | Line 117: | ||
==Lab manual pages== | ==Lab manual pages== | ||
| − | *[[Lab Manual:Optical Microscopy]] | + | *[[Lab Manual: Optical Microscopy]] |
| − | *[[ | + | **[[Optical Microscopy Part 1: Bright Field Microscopy]] |
| − | *[[ | + | **[[Optical Microscopy Part 2: Fluorescence Microscopy]] |
| − | *[[Optical Microscopy: | + | **[[Optical Microscopy: Measuring resolution using PSF beads]] |
| − | *[[Optical Microscopy: | + | **[[Optical Microscopy Part 3a: Particle Tracking in Glycerin Samples]] |
| − | *[[Optical Microscopy: Fluorescence Imaging of Actin Cytoskeleton]] | + | **[[Optical Microscopy Part 3b: Microrheology Measurements in Live Fibroblast Cells]] |
| − | + | **[[Optical Microscopy Part 3c: Fluorescence Imaging of Actin Cytoskeleton]] | |
| + | <br> | ||
==References== | ==References== | ||
Revision as of 22:58, 12 August 2013
| 1665 | 2009 |
|---|---|
|
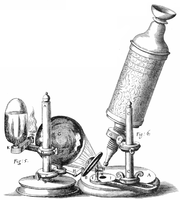
Robert Hooke's microscope |
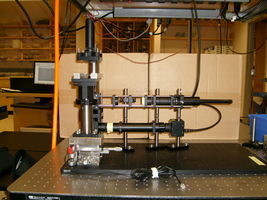
20.309 student's microscope |
|
Hooke micrograph of cork cells |
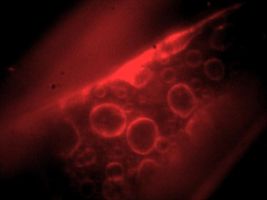
CCD image of fluorescent labeled intracellular membranes[1] |
I took a good clear piece of Cork, and with a Pen-knife sharpen'd as keen as a Razor, I cut a piece of it off, and thereby left the surface of it exceeding smooth, then examining it very diligently with a Microscope, me thought I could perceive it to appear a little porous; but I could not so plainly distinguish them, as to be sure that they were pores, much less what Figure they were of: But judging from the lightness and yielding quality of the Cork, that certainly the texture could not be so curious, but that possibly, if I could use some further diligence, I might find it to be discernable with a Microscope, I with the same sharp Penknife, cut off from the former smooth surface an exceeding thin piece of it, and placing it on a black object Plate, because it was it self a white body, and casting the light on it with a deep plano-convex Glass, I could exceeding plainly perceive it to be all perforated and porous, much like a Honey-comb, but that the pores of it were not regular; yet it was not unlike a Honey-comb in these particulars.
Don't you just buy a [expletive deleted] microscope?[3]
Introduction
Since Robert Hooke noticed that the pores in a very thin sample of cork reminded him of the small rooms called cells where monks sleep in a monastery, the optical microscope has been a tool of central importance. But Hooke did more than notice the sample's texture. He also made perhaps the first quantitative measurements of cells with an optical microscope:
I told several lines of these pores, and found that there were usually about threescore of these small Cells placed end-ways in the eighteenth part of an Inch in length, whence I concluded there must be neer eleven hundred of them, or somewhat more then a thousand in the length of an Inch, and therefore in a square Inch above a Million, or 1166400. and in a Cubick Inch, above twelve hundred Millions, or 1259712000. a thing almost incredible, did not our Microscope assure us of it by ocular demonstration.
— Robert Hooke[2]
Improvements in optical components, microscope designs, illuminators, imaging devices, and sample preparation methodologies fostered increasingly sophisticated discoveries in the three and a half centuries since Micrographia. Barbara McClintock observed genetic transposition through an optical microscope by in 1944, for example. She was a talented microscopist who developed a technique that let her visualize and differentiate individual chromosomes in Zea Mays (corn) plant cells. McClintock was awarded the Nobel Prize in Physiology or Medicine in 1983 for this discovery. It took several decades before molecular techniques sufficiently sophisticated to confirm her discovery were developed.[5]
In this lab, you will design and build an optical microscope from components. You will characterize the performance of your instrument and make images using two different contrast methodologies: transmitted brightfield and epifluorescence. Using particle tracking computer software, you will make quantitative measurements of small suspended particles in Brownian motion. Finally, you will use your microscope to make quantitative measurements of vesicle trafficking in live plant cells. (In particular, you will estimate the number of ATP molecules per second required for intracellular transport of vesicles.)
In past semesters, several student groups have undertaken final projects that involved expanding the capabilities of their microscope, including implementing scanning-sample confocal and darkfield contrast modes.
Objectives and Learning Goals
- Learn about the theory and practice of light microscopy
- Use ray tracing rules to design a transmitted bright field and fluorescent light microscope
- Construct the microscope from optical components
- Characterize the microscope's performance
- Adapt and create signal processing Matlab code for image analysis
- Track moving particles
- Make quantitative measurements of biological systems
- Identify your microscope's limits of detection, and understand their source(s)
- Record, enhance, and analyze microscope images
How to do this lab
Week 1: bright-field microscopy
- Read Part 1: Bright Field Microscopy
- Read the references; understand lenses, ray tracing, and magnification
- Design and build microscope
- Characterize the transmitted bright-field performance of the microscope
Week 2: fluorescence microscopy
- Read Part 2: Fluorescence microscopy
- Add a laser illumination beam path
- Characterize the fluorescent imaging performance of the microscope
- Image fluorescent samples
- Correct images for nonuniform illumination
Week 3+: experiments in fluorescence microscopy
- Read Part 3: Particle Tracking Applications of Optical Microscopy
- Image PSF beads and calculate resolution
- Track fixed beads and measure microscope stability. See Procedure: Particle tracking.
- Track microspheres suspended in a solvent
- Estimate diffusion coefficients in a Newtonian fluid; calculate viscosities
- Investigate the mechanical properties of the 3T3 cell cytoskeleton and cytoplasm
- Follow the protocols on the Microrheology Measurements via Particle Tracking and Fluorescence Imaging of Actin Cytoskeleton labeling wiki pages
How to report on this lab
For general guidelines, please see 20.309:Lab Report Guidelines.
For specific tasks and deliverables, please follow the guidelines of the suggested report outline.
Note that we are more likely to understand concise, clear prose. Less is more.
Microscopy lab etiquette
During the microscopy lab, approximately seven thousand optical components will be taken from stock, assembled into microscopes, and properly returnd to their assigned places. With the goal of keeping the frustration level to a minimum, please observe the following:
- Observe laser safety guidelines.
- Wear gloves when you are handling biological samples.
- Store your microscope in one of the cubby holes in 16-336. If you use one of the high shelves, get somebody to help you lift.
- Keep all of the boxes for the optics you use with your instrument to simplify putting things away.
- Take a blue bin to store loose items (such as lens boxes) in.
- Stages, CCD cameras, neutral density filters and barrier filters stay at the lab station. Do not store these with your microscope.
- Return objective lenses to the drawer when you are not using them. (Do not store them with your microscope.)
- The stages are very expensive. Always lift from the bottom.
- Ask a TA or instructor for help if you need to clean a dichroic or barrier filter. (These optics have exposed coatings and must be cleaned with extra care.)
- Check the focal length of lenses before you use them. Some students before you have been very bad at the three-of-these-things game. If you find an optic in the wrong box: identify the optic and replace it in the correct box or label the box correctly. (Ask an instructor or TA if you can't find the right box. There are many boxes near the wire spools behind you as you stand at the wet bench.)
- Never use an SM1T2 coupler without a locking ring — they are very difficult to remove if they are tightened against a lens tube or tube ring.
- Use tube rings (never an SM1T2, SM1V01, or SM1V05) to mount optics in lens tubes.
- If you break something (or discover something pre-broken for you), do not return it to the component stock. Give all broken items to an instructor or TA. You will not be penalized for breaking something, but not reporting may be looked upon less kindly.
- Please do not remove parts from the TA microscope
- Each group will receive their own LED and dichroic mirror. Please ask a TA if you cannot find one.
- Many students have had trouble with where to begin as they design the microscope. Read through this lab manual carefully, many of the design constraints and tradeoffs have been outlined.
- A good plan will often save you time and headaches down the road. If you are not sure if your plan is a good one, talk to an instructor. We are here to help.
Lab manual pages
- Lab Manual: Optical Microscopy
- Optical Microscopy Part 1: Bright Field Microscopy
- Optical Microscopy Part 2: Fluorescence Microscopy
- Optical Microscopy: Measuring resolution using PSF beads
- Optical Microscopy Part 3a: Particle Tracking in Glycerin Samples
- Optical Microscopy Part 3b: Microrheology Measurements in Live Fibroblast Cells
- Optical Microscopy Part 3c: Fluorescence Imaging of Actin Cytoskeleton
References
- ↑ Onion endothelial cell incubated with FM 4-64 dye (Invitrogen). See class stellar site for protocol. Oh & Yamaguchi, unpublished lab report
- ↑ 2.0 2.1 Hooke, R. Micrographia: or Some Physiological Descriptions of Minute Bodies made by Magnifying Glasses with Observations and Inquiries Thereupon London:Jo. Martyn, and Ja. Allestry, Printers to the Royal Society; 1665
- ↑ http://www.historycommons.org/context.jsp?item=a043074editedtranscripts
- ↑ Fall 2007
- ↑ See, for example: McClintock, B. The origin and behavior of mutable loci in maize. PNAS. 1950; 36:344-355. [1], [2], and Endersby, Jim. A Guinea Pig's History of Biology. Cambridge, Massachusetts: Harvard University Press; 2007.