Difference between revisions of "Assignment 9, Part 1: Analyze two-color yeast images"
Juliesutton (Talk | contribs) (→Written questions) |
Juliesutton (Talk | contribs) (→Analyze a frame) |
||
| Line 38: | Line 38: | ||
{{Template:Assignment Turn In|message = Choose a single frame of the movie. Make a four panel figure and display: the blue image, the green image (both with a scale bar), and their corresponding image histograms. Include an appropriate title for each panel. }} | {{Template:Assignment Turn In|message = Choose a single frame of the movie. Make a four panel figure and display: the blue image, the green image (both with a scale bar), and their corresponding image histograms. Include an appropriate title for each panel. }} | ||
| − | == | + | ===Estimating background levels=== |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
[[File:BlueImageHistogram.png|thumb|right]] | [[File:BlueImageHistogram.png|thumb|right]] | ||
| − | It's tricky to get good images of proteins expressed by living yeast cells. Not only do we need to use very high exposure times (on the order of 5 s) to capture a dim signal, but we want to image cells over long times (minutes to hours). These | + | It's tricky to get good images of fluorescent proteins expressed by living yeast cells. Not only do we need to use very high exposure times (on the order of 5 s) to capture a dim signal, but we want to image cells over long times (minutes to hours). These factors work against us to produce high background levels caused by autofluorescence of the surrounding medium, camera noise (especially dark noise), and potentially light leakage from the room. Any drift in the background level over time can obscure our results. Additionally, the background level may not be uniform over the entire image. For each frame of the movie, we will subtract off a background image (taken with no cells present) which has been scaled appropriately to account for changing levels over time. |
| − | In | + | In the example data provided, you should find a background image taken with no yeast present - only background coming from the medium autofluorescence and other background sources. To determine how much we need to scale the image, we will compare the background image intensity to an empty region of our cell images. |
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| + | Use this function to locate an empty region of the first frame in your movie: | ||
<pre> | <pre> | ||
| − | function | + | function BacgroundRectangle = LocateBacgroundRegion( InitialYeastImage ) |
| − | |||
figure | figure | ||
| − | + | imshow( InitialYeastImage(:,:,1) ) | |
| − | + | title('Drag a rectangle around a region of the image containing only background') | |
| + | BacgroundRectangle = getrect( gcf ); | ||
| + | close | ||
| − | + | end | |
| − | + | </pre> | |
| − | + | ||
| − | + | Then for each frame, you can calculate the scale factor needed for your background image: | |
| − | + | <pre> | |
| − | + | function bacgroundScaleFactors = FindBacgroundScaleFactor( InitialImage, BacgroundImage, BacgroundRectangle ) | |
| − | + | bacgroundScaleFactors = nan(1,2); | |
| − | + | % Scale background | |
| − | + | for j = 1:2 | |
| − | + | backgroundImageCropped = imcrop(BacgroundImage(:,:,j),BacgroundRectangle); | |
| − | + | initialImageCropped = imcrop(InitialImage(:,:,j),BacgroundRectangle); | |
| − | + | bacgroundScaleFactors(j) = mean(initialImageCropped(:))/ mean(backgroundImageCropped(:)); | |
| − | + | end | |
| − | + | ||
end | end | ||
</pre> | </pre> | ||
| − | {{Template:Assignment Turn In|message = | + | Test the code by finding the background scale factors for the first frame of the GFP and RFP images. Try using several different rectangular regions and notice how much the output varies. |
| + | {{Template:Assignment Turn In|message = Display an example of a background-subtracted and contrast-stretched image for a GFP and RFP image.}} | ||
| − | == | + | ==Analyze a frame== |
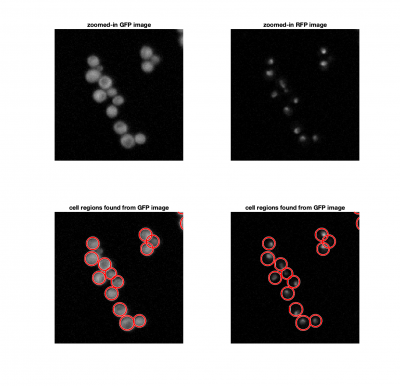
| − | + | In each frame of our movie, we need to locate and identify individual cells, and find the correlation coefficient between the cell image and the nuclei image. If the Hog1 signal is localized in the nucleus, the correlation should be close to 1. | |
| + | |||
| + | [[Image:FindingCellsAndNucleiExample.png|center|thumb|400px]] | ||
| + | |||
| + | Start with the following basic layout of the function: | ||
<pre> | <pre> | ||
| − | + | function NucleusHog1Correlation = AnalyzeFrame( TwoColorFrame, BackgroundImage ) | |
| − | + | ||
| + | % 1. estimate the background level | ||
| + | % 2. identify individual yeast cells in the GFP image | ||
| + | % 3. calculate the correlation coefficient between the GFP intensities an RFP intensities within each cell region | ||
| + | % 4. output the mean correlation coefficient for all cells in the frame | ||
| + | |||
| + | end | ||
</pre> | </pre> | ||
| − | + | As you work through this part of the Assignment, fill out the <tt>AnalyzeFrame</tt> function to incorporate each of the outlined steps. | |
===Finding cells=== | ===Finding cells=== | ||
| − | We could | + | We could consider using a threshold to identify cells in an image, similar to how we found fluorescent particles in Assignments 4 and 5. The problem here, is that thresholding doesn't allow us separate a single cells from a clump of cells. Since we know that yeast cells are roughly spherical, we can use <tt>imfindcircles</tt> to locate them. <tt>imfindcircles</tt> is an implemenattion of the [https://www.mathworks.com/help/images/ref/imfindcircles.html| Hough transform]. |
| − | + | ||
| − | + | ||
<pre> | <pre> | ||
| Line 119: | Line 105: | ||
</pre> | </pre> | ||
| − | + | Here, <tt>Rmin</tt> and <tt>Rmax</tt> are the smallest and largest expected 'radii'. How can you estimate <tt>Rmin</tt> and <tt>Rmax</tt>? | |
| + | |||
=== Loop through cells and calculate <GFP_cell> and <GFP_nucleus> === | === Loop through cells and calculate <GFP_cell> and <GFP_nucleus> === | ||
| + | |||
| + | \*****WARNING: there is an alternate way to do this section that is more reliable. The wiki will be updated to reflect the new method on Monday, 4/21/2019******* | ||
Now that we've found our cells and nuclei we need to loop through each cell, and calculate the GPF intensity inside and out of the nucleus. | Now that we've found our cells and nuclei we need to loop through each cell, and calculate the GPF intensity inside and out of the nucleus. | ||
Revision as of 19:53, 21 April 2019
Written questions
| |
Read the paper and answer the written questions in Assignment 9 Overview: Analyzing yeast images. |
Yeast image analysis code
Start by downloading Assignment9TestData.mat. This is an example of the data you will be collecting in assignment 10 with an 8 minute valve oscillation period. The movie was collected using the same 20.309 microscope that you built in Assignment 8. During an oscillation experiment, the microscope first recorded an image with blue illumination followed by one with green illumination. It had a 40x objective and a 125 mm tube lens - remember that results in a 25X magnification.
The sample is similar to the one in Mettetal et al.: we are using S. cerevisiae with the protein Hog1 fused to GFP (which is excited by blue light) and an mRNA binding protein (we'll call MCP) fused to tagRFP (which is excited by green light) to locate the nucleus. Our goal is to calculate the average Hog1 intensity in the nucleus (<GFP_nucleus>) vs. the whole cell (<GFP_cell>) as a function of the high/low valve state.
In MATLAB, display the contents of the structure in the data file:
twoColorYeastTest =
struct with fields:
Movie: [544×728×2×16 double]
Time: [16×2 double]
ValveOscillationPeriod: 480
BlueCameraGainAndExposure: [5 5000000]
GreenCameraGainAndExposure: [15 5000000]
ValveState: [16×1 logical]
The data structure contains all the important information from the experiment:
- A two-color movie, where the 3d dimension is the color (1 = Blue, 2 = Green), and the fourth is the frame number. Notice that twoColorYeastTest.Movie has already converted to a double, but it is not yet normalized by 4095.
- The time stamp of each frame, recorded in seconds from the start of the experiment. Column 1 contains the timestamps of the Blue images, column 2 is for the green images.
- The oscillation valve period, in seconds.
- The blue and green image camera settings in the format [gain, exposure], where exposure is in microseconds.
- The valve state (0 for low salt, 1 for high salt) at the time of each image. If you need to know the valve state more precisely, it can be calculated with the following conditional statement: $ sin(2 \pi t/T) \ge 0 $, where T is the valve oscillation period, and t is the time since the start of the experiment (this is how the state is determined in the software).
Estimating background levels
It's tricky to get good images of fluorescent proteins expressed by living yeast cells. Not only do we need to use very high exposure times (on the order of 5 s) to capture a dim signal, but we want to image cells over long times (minutes to hours). These factors work against us to produce high background levels caused by autofluorescence of the surrounding medium, camera noise (especially dark noise), and potentially light leakage from the room. Any drift in the background level over time can obscure our results. Additionally, the background level may not be uniform over the entire image. For each frame of the movie, we will subtract off a background image (taken with no cells present) which has been scaled appropriately to account for changing levels over time.
In the example data provided, you should find a background image taken with no yeast present - only background coming from the medium autofluorescence and other background sources. To determine how much we need to scale the image, we will compare the background image intensity to an empty region of our cell images.
Use this function to locate an empty region of the first frame in your movie:
function BacgroundRectangle = LocateBacgroundRegion( InitialYeastImage )
figure
imshow( InitialYeastImage(:,:,1) )
title('Drag a rectangle around a region of the image containing only background')
BacgroundRectangle = getrect( gcf );
close
end
Then for each frame, you can calculate the scale factor needed for your background image:
function bacgroundScaleFactors = FindBacgroundScaleFactor( InitialImage, BacgroundImage, BacgroundRectangle )
bacgroundScaleFactors = nan(1,2);
% Scale background
for j = 1:2
backgroundImageCropped = imcrop(BacgroundImage(:,:,j),BacgroundRectangle);
initialImageCropped = imcrop(InitialImage(:,:,j),BacgroundRectangle);
bacgroundScaleFactors(j) = mean(initialImageCropped(:))/ mean(backgroundImageCropped(:));
end
end
Test the code by finding the background scale factors for the first frame of the GFP and RFP images. Try using several different rectangular regions and notice how much the output varies.
| |
Display an example of a background-subtracted and contrast-stretched image for a GFP and RFP image. |
Analyze a frame
In each frame of our movie, we need to locate and identify individual cells, and find the correlation coefficient between the cell image and the nuclei image. If the Hog1 signal is localized in the nucleus, the correlation should be close to 1.
Start with the following basic layout of the function:
function NucleusHog1Correlation = AnalyzeFrame( TwoColorFrame, BackgroundImage )
% 1. estimate the background level
% 2. identify individual yeast cells in the GFP image
% 3. calculate the correlation coefficient between the GFP intensities an RFP intensities within each cell region
% 4. output the mean correlation coefficient for all cells in the frame
end
As you work through this part of the Assignment, fill out the AnalyzeFrame function to incorporate each of the outlined steps.
Finding cells
We could consider using a threshold to identify cells in an image, similar to how we found fluorescent particles in Assignments 4 and 5. The problem here, is that thresholding doesn't allow us separate a single cells from a clump of cells. Since we know that yeast cells are roughly spherical, we can use imfindcircles to locate them. imfindcircles is an implemenattion of the Hough transform.
[cellCenters, cellRadii] = imfindcircles(cellFrame,[Rmin Rmax],'ObjectPolarity','bright','Sensitivity',0.95);
Here, Rmin and Rmax are the smallest and largest expected 'radii'. How can you estimate Rmin and Rmax?
Loop through cells and calculate <GFP_cell> and <GFP_nucleus>
\*****WARNING: there is an alternate way to do this section that is more reliable. The wiki will be updated to reflect the new method on Monday, 4/21/2019*******
Now that we've found our cells and nuclei we need to loop through each cell, and calculate the GPF intensity inside and out of the nucleus.
Here's a function to get you started:
function allCellProperties = FindCellularAndNuclearHog1Intensities(CellFrame, CellCenters, CellRadii, NucleiMask, enablePlotting)
if nargin<5
enablePlotting = true;
end
if enablePlotting
figure
imshow(CellFrame)
hold on
visboundaries(createMaskFromCircles(CellCenters,CellRadii,size(CellFrame)),'Color','r');
visboundaries(NucleiMask,'Color','w');
title('found cells and nuclei')
end
numCellsInFrame = length(CellRadii);
maskImageSize = size(NucleiMask);
allCellProperties = cell(0);
% loop through each individual cell
for k = 1:numCellsInFrame
% create a mask for a single cell.
cellMask =
% create a mask of that cell's nucleus
nucleusMask =
% is this a good cell?
% choose a criteria to filter out bad cells. some cases to consider
% are: does the cell have a nucleus? is the nucleus too big or too
% small?
isGoodCell =
if isGoodCell
if enablePlotting
visboundaries(cellMask,'Color','b')
visboundaries(nucleusMask,'Color','g')
end
% use regionprops to to calculate the mean intensity of the nucleus
% and of the whole cell
% store the properties in a struct...
singleCellProperties = struct();
% ... and append the struct to a cell array to store all your data.
allCellProperties{end+1} = singleCellProperties;
end
end
end
| |
Write the rest of FindCellularAndNuclearHog1Intensities so that it outputs a cell array containing the average GFP intensities inside the nucleus, and for the whole yeast cell. |
You may use the following helper function:
function Im = createMaskFromCircles(centers,radii,ImageSize)
Im = zeros(ImageSize);
[X, Y] = meshgrid(1:ImageSize(2), 1:1:ImageSize(1));
for ii = 1:length(radii)
isBright = (X-centers(ii,1)).^2+(Y-centers(ii,2)).^2 <= radii(ii,1)^2;
isAlreadyFound = (Im == 1);
Im = Im + (isBright & ~isAlreadyFound);
end
Im( Im > 1 ) = 1;
end
Calculating the response of Hog1
In Mettetal et al., they define the Hog 1 response as the average fluorescence of Hog1 inside the nucleus normalized by the total cell fluorescence. We also need to ensure to remove our background:
$ R_{Hog1} = \frac{<GFP_{nucleus}> - <GFP_{background}>}{<GFP_{cell}> - <GFP_{background}>} $
Hint: You may find the cellfun function useful for accessing an element from a cell array of structs:
outputArray = cellfun(@(c) c.structElement, yeastProperties);
Analyze the movie
Once you've set up your analysis for a single frame, you'll now want to loop through the movie and collect the responses as a function of time.
| |
Turn in all your MATLAB code in pdf format. No need to include functions that you used but did not modify. |
- Overview
- Part 1: Analyze a two-color yeast movie frame
Back to 20.309 Main Page