Difference between revisions of "20.109(S24):M2D1"
Becky Meyer (Talk | contribs) (→Part 3: Examine data for ΔMet17 yeast cell surface amino acids) |
Becky Meyer (Talk | contribs) (→Part 3: Examine data for ΔMet17 yeast cell surface amino acids) |
||
| Line 56: | Line 56: | ||
<font color = #4a9152 >'''In your laboratory notebook,'''</font color> complete the following: | <font color = #4a9152 >'''In your laboratory notebook,'''</font color> complete the following: | ||
*Which peptide sequence is your choice? | *Which peptide sequence is your choice? | ||
| − | *What is your rationale for choosing this peptide sequence | + | *What is your rationale for choosing this peptide sequence? |
===Part 4: Choose a peptide sequence and determine a DNA sequence to encode it === | ===Part 4: Choose a peptide sequence and determine a DNA sequence to encode it === | ||
Revision as of 19:48, 10 March 2024
Contents
Introduction
Heavy metals are typically thought of as metal elements with a relatively high density. Many of these metals are considered precious (gold, silver, platinum) or have commercial value (lead, copper, nickel). Other metals can play an essential health role in small quantities (zinc, iron, manganese). However many of these metals are toxic, even when present in parts per billion. Heavy metals can be released into the environment through a number of activities such as mining, industrial production of consumer products, or disposal of electronic waste.
In this module, you will focus on protein engineering of a cell surface display peptide in Saccharomyces cerevisiae, also known as baker's yeast. We are using this YSD (yeast cell surface display) model to capture the heavy metal cadmium in a recyclable form. This type of protein engineering is an early step in optimizing a "bioremediation" model system. Bioremediation is an approach to cleaning up environmental pollutants by repurposing a known biological process. Typically, bioremediation utilizes microbial and fungal organisms to remove environmental contaminants as these single cell organisms can be fast growing and genetically tractable (i.e. relatively simple to genetically manipulate).
Today you examine what is known about our current S.cerevisiae bioremediation model system. You will explore the genetic metabolic engineering used to induce the yeast to produce hydrogen sulfide, examine the components of yeast cell surface display, and examine previously expressed peptides. You will use this information to rationally design a peptide to express on the yeast cell surface in order to capture Cadmium particles in the most uniform distribution possible. The rest of the module will be devoted to expressing your chosen peptides in our yeast model system, and testing these peptides for their suitability in bioremediation.
Protocols
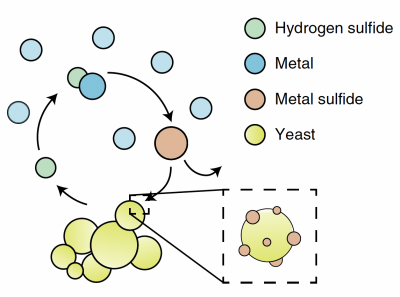
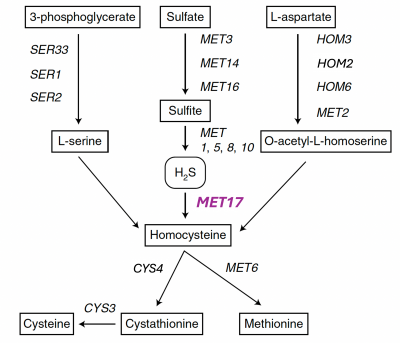
Part 1: Review metabolic engineering approach to create ΔMet17 yeast
In this module,
In your laboratory notebook, complete the following:
- How
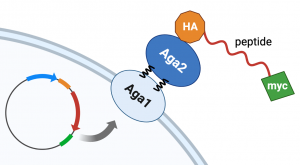
Part 2: Review yeast cell surface display
In this module, we will use yeast cell surface display to express a peptide which can capture precipitating cadmium sulfide
In your laboratory notebook, complete the following:
- Based
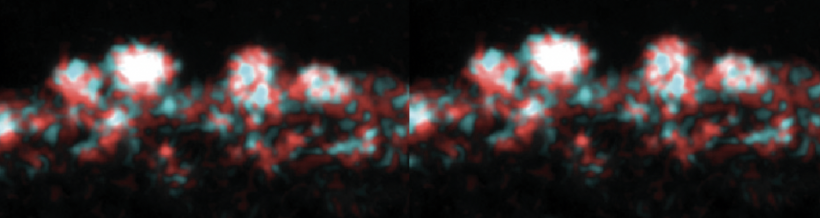
Part 3: Examine data for ΔMet17 yeast cell surface amino acids
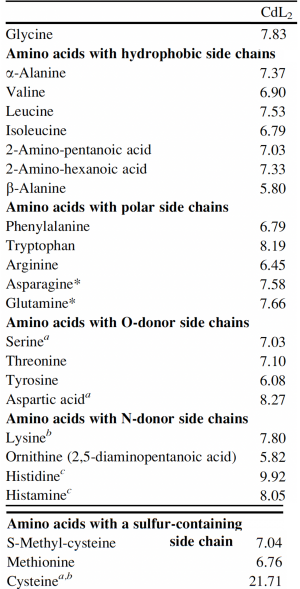
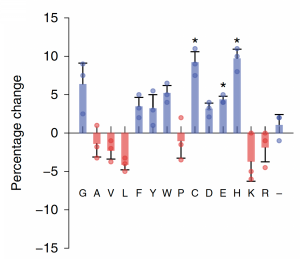
Now that you have noted relevant information regarding the ΔMet17 YSD model system, it's time to turn your attention to our metal of interest, cadmium. While there is limited information on the specific mechanism of cadmium capture by peptides, previous literature has indicated that amino acid residues form complexes of differing stability with cadmium. Using the table to the right, examine the reported affinities of cadmium for different amino acid residues. In previous work from the Belcher lab, the ability of particular amino acid residues to remove cadmium from spiked media was tested using yeast display. The results of that experiment are shown at right and detailed in Sun et. al, 2020 (linked on the Mod2 landing page).In your laboratory notebook, complete the following:
- Which amino acids seem like likely candidates to capture precipitating cadmium?
- Which amino acids seem to be somewhat capable of capturing cadmium (the mid-range candidates)?
- Which amino acids have the lowest likelihood of capturing cadmium?
After looking at these data, you may be convinced that a string of cysteine residues are an excellent way to capture cadmium. This is indeed true. As the Belcher lab has found, a hexapeptide of cysteine repeats will effectively capture cadmium in solution. However, they also found that the cadmium sulfide particles which bind to cysteine-only peptide motifs form more amorphous cadmium sulfide deposits than a peptide motif which included glycine residues (Gly-Cys-Cys-Gly-Cys-Cys). Our goal in this module is to capture cadmium in a usable form, with crystalline features that produce a strong fluorescent signal, so that we can recycle captured cadmium into valuable quantum dots. The Belcher lab has found that if the cadmium sulfide precipitates rapidly, it will form amorphous structure. However, if the cadmium sulfide precipitates at a more moderate pace and is captured at a more moderate pace, it will be able to form more organized structures which ultimately emit a stronger fluorescent signal.
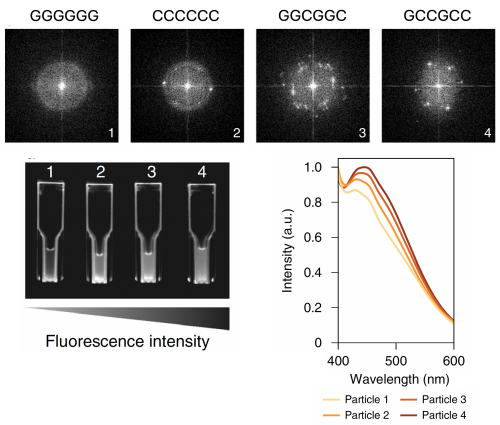
In order to design a cell surface display peptide that is capable of capturing precipitating sulfide in ordered particle formation, you will need to consider the:
- Amino acid residue binding to cadmium sulfide
- Sequence of amino acids in the peptide
- Length of peptide
- The Belcher lab chose to display hexapeptides, however you can choose between 1-8 amino acids to comprise your displayed peptide.
With your lab partner, use these parameters to determine the peptide you would like to display on the surface of ΔMet17 yeast to capture precipitating cadmium for recycling.
In your laboratory notebook, complete the following:
- Which peptide sequence is your choice?
- What is your rationale for choosing this peptide sequence?
Part 4: Choose a peptide sequence and determine a DNA sequence to encode it
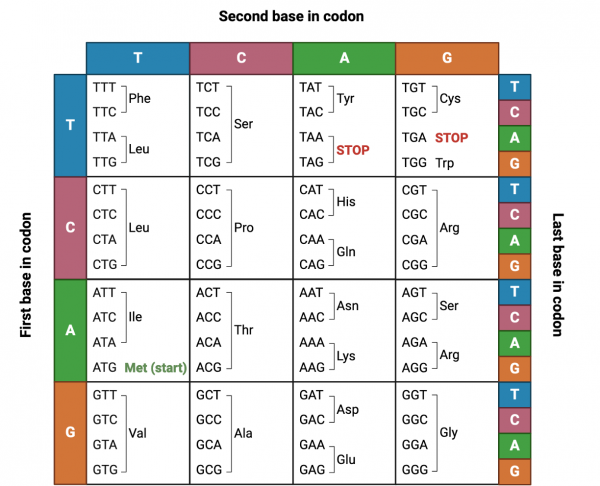
Using the information you have gathered above, you can now determine what peptide you would like express in order to capture precipitating cadmium. Using the DNA sequences corresponding to each nucleotide, you can create sequences to become primers for use in sit-directed mutagenesis cloning to insert your sequence in the yeast display vector. Although the process of choosing the DNA sequence for amino acid codons is straightforward, certain codons can be created from more than one DNA sequence. Here is where you will want to consider technical strategy in cloning.
The instructors will take your desired DNA sequence and flank it with additional oligos to orient it correctly in the pCTCON2 vector. In so doing, we will be able to address most of the parameters listed below for successful primer design.
Successful DNA primers tend to follow the following parameters:
- Primers should be 25-45 bp long.
- G/C content of 40-60% is desired.
- Both primers should terminate in at least one G or C base.
- Melting temperature should exceed 78°C, according to:
- Tm = 81.5 + 0.41 (%GC) – 675/N - %mismatch
- where N is primer length and the two percentages should be integers
It is helpful for your chosen DNA sequence to not be exceptionally GC rich so that we can keep an overall GC content of primers to 40-60%. This is worth keeping in mind when you are choosing between different potential sequences for the amino acid codon you want to encode.
In your laboratory notebook, complete the following:
- What amino acid sequence have you decided?
- What DNA sequence have you chosen to encode your peptide?
Determine the DNA sequence you need to encode your amino acids and upload it to the Class Data page on the wiki before you leave.
- These primers must be ordered by 6pm to arrive in time for your next experiment.
- Anchor sequences will be added to your primers to orient the new sequence between the peptide tags. These sequences will be detailed in the next lab.
Next day: Perform site-directed mutagenesis