Difference between revisions of "20.109(S16):Complete Western and prepare damaged DNA (Day3)"
Noreen Lyell (Talk | contribs) (→Measuring protein concentration) |
Noreen Lyell (Talk | contribs) (→Measuring protein concentration) |
||
| Line 25: | Line 25: | ||
Traditionally, only one species of antibody could be used on a Western blot because the detection relied on the emission of light that was collected by x-ray film. In the traditional systems the output looks like black bands on a blue or clear background. However, more recent conjugate chemistry has allowed secondary antibodies to be coupled to fluorescent tags. Today we will use infrared (IR) secondary antibodies to detect our α-DNA-PK and α-tubulin antibodies and then scan the Western blots using a specially constructed microscope located in the Lauffenburger lab to determine the level of DNA-PK in our cell lines. | Traditionally, only one species of antibody could be used on a Western blot because the detection relied on the emission of light that was collected by x-ray film. In the traditional systems the output looks like black bands on a blue or clear background. However, more recent conjugate chemistry has allowed secondary antibodies to be coupled to fluorescent tags. Today we will use infrared (IR) secondary antibodies to detect our α-DNA-PK and α-tubulin antibodies and then scan the Western blots using a specially constructed microscope located in the Lauffenburger lab to determine the level of DNA-PK in our cell lines. | ||
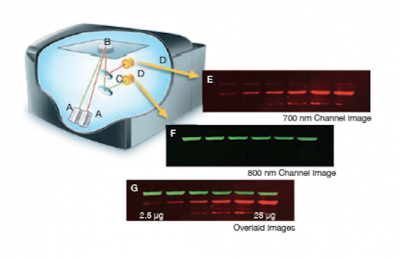
| − | [[Image:Sp16 M2D3 licor imaging.png|thumb|400px| right| '''Licor detection of | + | [[Image:Sp16 M2D3 licor imaging.png|thumb|400px| right| '''Licor detection of IR-dye conjugated antibodies.''' Image was modified from the Odyssey manual.]]The Licor Odyssey scanner consists of an inverted microscope with two lasers that excite dyes which emit light in the IR range. As depicted in the image on the right, an excitation point is created when beams from the 700 nm and 800 nm lasers (A) are focused on the scanning surface. The microscope objective (B) is focused on the excitation point and collects light from the fluorescing IR dyes. This light is passed through a dichroic mirror (C) that separates the light into two distinct signals that travel through two independent optical paths that are focused on separate silicon photodiodes (D) and detected. In the image, the first channel (E) and second channel (F) are shown separately and merged (G). |
We will detect our IR-dye conjugated secondary antibodies at wavelengths of 700 and 800 nm. The 700 nm channel will appear red and the 800 nm channel will appear green. Infrared secondary antibodies provide a more flexible detection platform than the traditional Western blot detection methods that rely on [http://en.wikipedia.org/wiki/Western_blot#Colorimetric_detection colorimetric] or [http://www.lifetechnologies.com/us/en/home/life-science/protein-biology/protein-biology-learning-center/protein-biology-resource-library/pierce-protein-methods/chemiluminescent-western-blotting.html chemiluminescent] substrates. Unlike the colorimetric or chemiluminescent detection methods, IR dyes do not require a chemical reaction to occur in order for signal to be detected. This means that the output signal increases with time as the colorimetric or chemiluminescent substrate reaction proceeds -- making timing an important variable in traditional Western blot development. We remove that variable from the equation and control when we want to visualize our Western blot simply by controlling the excitation of the dye. | We will detect our IR-dye conjugated secondary antibodies at wavelengths of 700 and 800 nm. The 700 nm channel will appear red and the 800 nm channel will appear green. Infrared secondary antibodies provide a more flexible detection platform than the traditional Western blot detection methods that rely on [http://en.wikipedia.org/wiki/Western_blot#Colorimetric_detection colorimetric] or [http://www.lifetechnologies.com/us/en/home/life-science/protein-biology/protein-biology-learning-center/protein-biology-resource-library/pierce-protein-methods/chemiluminescent-western-blotting.html chemiluminescent] substrates. Unlike the colorimetric or chemiluminescent detection methods, IR dyes do not require a chemical reaction to occur in order for signal to be detected. This means that the output signal increases with time as the colorimetric or chemiluminescent substrate reaction proceeds -- making timing an important variable in traditional Western blot development. We remove that variable from the equation and control when we want to visualize our Western blot simply by controlling the excitation of the dye. | ||
Revision as of 19:30, 17 February 2016
Introduction
Measuring NHEJ repair
In Module 1 we discussed two used for restriction enzymes: digesting DNA fragments in preparation for ligation and digesting DNA products to confirm cloning. Here we will use restriction enzymes to generate damaged DNA for your NHEJ assay. Specifically, the damaged DNA will serve as a substrate for NHEJ in the M059K and M059J cells. Because dsb repairs result in a flourescent signal, you will use a flow cytometer to count the number of cells that successfully repaired the damage caused by the restriction enzyme(s). Moreso than just measuring NHEJ activity in the cell lines, you will also investigate the impact of the type of DNA damage on NHEJ repair.
You will begin today by digesting pMAX-EGFP-MCS using the restriction enzyme(s) you selected previously. Introducing restriction enzyme site-based damage into DNA is just one creative way that scientists and engineers have harnessed the power of nature. In both Module 1 and this current module, you have witnessed molecules (such as ligase) and cells (such as E. coli) stripped from their natural context and applied to solve a problem. Though it now seems mundane, this practice of purifying and re-engineering biology for human ends is pretty amazing if you think about it! Several of the enzymes that will be used in class are “high fidelity” variants, designed for more specific activity than nature requires. Other enzymes have been engineered for faster kinetics.
The way that we are using restriction enzymes in Module 2, while not unprecedented, is somewhat unusual. You already performed the two far more common laboratory practices that utilize restriction enzymes. One is restriction-based cloning of distinct pieces of DNA with compatible ends. Remember, an insert and vector are ligated together, and then amplified in bacteria. Also, individual DNA clones are miniprepped and tested for correctness. In this second practice, restriction enzymes are just as useful in confirmation or diagnostic digests as they are in creating a clone in the first place. You might be wondering why researchers go through the trouble of designing and performing diagnostic digests, when sequencing is relatively simple and yields more information. Here, the idea of scale becomes important. Sequencing costs $6-8 per reaction, which can add up if you need to examine, say, 10 or more candidates. Agarose gel electrophoresis, by comparison, costs perhaps $1 per candidate. Since both methods require DNA isolation, one is not dramatically more labor intensive than the other. Finally, banding patterns can give a quick readout of many candidate colonies at once compared to the time it takes for individual sequencing analyses (at least if performed manually).
After digesting pMAX-EGFP-MCS to generate damaged ends, you will evaluate the completeness of the digestion on a gel. This step will ensure that the plasmid was digested and also allow you to separate the plasmid from the nonsense insert via gel purification. You already know plenty about agarose electrophoresis, and a fair bit about purifying DNA as well. In gel purification, the agarose containing the DNA of interest is excised from the gel and melted. The DNA is then isolated on a silica (SiO2) column similar to the ones you used in Module 1. Salt concentration and pH effects, along with ethanol precipitation, will alternately allow for binding and eluting the DNA while washing away contaminants.
Measuring protein concentration
In addition to preparing your damaged DNA for the NHEJ repair assay, you will also complete your Western blot analysis. Your blot was probed with two primary antibodies overnight by the teaching faculty - α-DNA-PK and α-α-tubulin. The α-DNA-PK antibody was raised in a mouse and the α-α-tubulin antibody was raised in a rabbit. Today you will add the two secondary antibodies that will allow for detection.
Traditionally, only one species of antibody could be used on a Western blot because the detection relied on the emission of light that was collected by x-ray film. In the traditional systems the output looks like black bands on a blue or clear background. However, more recent conjugate chemistry has allowed secondary antibodies to be coupled to fluorescent tags. Today we will use infrared (IR) secondary antibodies to detect our α-DNA-PK and α-tubulin antibodies and then scan the Western blots using a specially constructed microscope located in the Lauffenburger lab to determine the level of DNA-PK in our cell lines.
The Licor Odyssey scanner consists of an inverted microscope with two lasers that excite dyes which emit light in the IR range. As depicted in the image on the right, an excitation point is created when beams from the 700 nm and 800 nm lasers (A) are focused on the scanning surface. The microscope objective (B) is focused on the excitation point and collects light from the fluorescing IR dyes. This light is passed through a dichroic mirror (C) that separates the light into two distinct signals that travel through two independent optical paths that are focused on separate silicon photodiodes (D) and detected. In the image, the first channel (E) and second channel (F) are shown separately and merged (G).We will detect our IR-dye conjugated secondary antibodies at wavelengths of 700 and 800 nm. The 700 nm channel will appear red and the 800 nm channel will appear green. Infrared secondary antibodies provide a more flexible detection platform than the traditional Western blot detection methods that rely on colorimetric or chemiluminescent substrates. Unlike the colorimetric or chemiluminescent detection methods, IR dyes do not require a chemical reaction to occur in order for signal to be detected. This means that the output signal increases with time as the colorimetric or chemiluminescent substrate reaction proceeds -- making timing an important variable in traditional Western blot development. We remove that variable from the equation and control when we want to visualize our Western blot simply by controlling the excitation of the dye.
Protocols
Today you get to experience grad student life, juggling multiple assays with staggered incubation times. Be sure not to forget about your digesting DNA while completing your Western, or about your developing Western while gel purifying your DNA!
Part 1: Prepare damaged DNA for NHEJ assay
- You will digest pMax-BFP-MCS at the cut site(s) that you chose to investigate last time. We will then evaluate and purify the DNA using gel electrophoresis.
- To avoid pipetting very small volumes, you will either prepare a reaction cocktail that uses no less than 1 μL of any restriction enzyme, or you will prepare an intermediate dilution of said enzyme(s).
- See the M2D2 homework and/or talk to your instructors for more details!
- Note that enzyme stock concentrations can be found on the NEB product page for that enzyme.
- By whichever approach outlined above, combine 7.0 μg of DNA with water, buffer, and enzyme in a well-labeled eppendorf tube. Whether you prepare an enzyme dilution or a master mix, the enzyme should be added last.
- Why? What would happen if you added the enzyme directly to water?
- Recall that you are using 2.5 U of each enzyme per μg of DNA.
- Flick the tubes to mix the contents, touch-spin, then incubate the mixtures at 37°C for at least one hour. Write down your start time and also set a timer, in case you get distracted later on.
- While your samples are digesting, you can finish the Western. (Or at least start finishing it!)
Part 2: Complete Western blot protein analysis
Last time, you prepared protein extracts from K1 and xrs6 cells, separated them by SDS-PAGE, and transferred them to a nitrocellulose membrane. On that day, blots were moved to blocking buffer. The next day, they were incubated with primary antibodies against Ku80 and α-Tubulin overnight at 4 °C. (See reagent list at end of lab for concentrations/compositions/etc.)
- Obtain your blots from the front bench. Pour the antibody solution into a conical tube, writing the identity of the antibodies and the date on the tube.
- Because the antibody is in excess, sometimes the primary solution may be re-used on another blot. Worth saving until we see how our Westerns come out today, at least!
- Add enough TBS-T to cover your membranes – between 10-15 mL should work, but you don't need to measure out this volume. Keep in mind that the washing steps work by dilution, so it is a balance between adding enough to create a sink for the primary antibody, but not so much that you make a huge mess on the shaker!
- TBS-T stands for Tris-buffered saline with 0.1% Tween 20 (a surfactant).
- Shake your container for 5 min at 80 rpm, using the room temperature shaker
- Repeat for a total of 6 washes.
- Just before pouring off the last wash, prepare the secondary antibodies.
- Dilute the secondary antibodies in 10 mL of Blocking Buffer. They are light sensitive so find them on the front bench next to the Blocking Buffer and then wrap your tube in aluminum foil.
- For Ku80 (Rabbit)/ α-Tubulin (Mouse) -- use the anti-Rabbit IR680 (RED) antibody at 1:10,000 + anti-Mouse IR800 (GREEN) at 1:10,000
- After the last wash, add your secondary antibody solution, place on the room temperature shaker, and cover your western blot container with aluminum foil
- Shake at 65 rpm for about 45 minutes
- Pour off the secondary antibody
- Wash the membrane at 80 rpm, using the room temperature shaker, with TBS-T for 3 washes at 7 minutes each
- After removing the last TBS-T wash, rinse the membranes 1x with 25 mL PBS. Pour off the PBS and keep in another 25mL of PBS until you can scan the membrane.
- The Odyssey scanner is located in the Lauffenburger lab in room 56-378, one of the teaching staff will accompany you there in groups to scan your blots.
Part 3: Gel purify damaged DNA for NHEJ assay
- When your digest is ready – and you are, too – add 5 μL of 6x NEB loading dye to it, and then pipet 27 μL (or however much you end up with after pipetting error losses) into a 1% gel according to the scheme in the table below.
- The gels will be run for about 30 minutes at 100 V, which should give sufficient separation between the plasmid fragment of interest and the nonsense fragment.
- At least 10 minutes before your gel run is slated to be over, label and weigh an eppendorf tube.
- I usually find it easiest to write the value right on the tube, especially if I am measuring multiple weights.
- After your gel run is finished, the teaching faculty will show you how to safely cut the band out of your gel.
Three groups can fit on one gel. We are leaving space between the samples for two reasons:
- We don't want the differently digested DNA bands to bleed into each other, but rather to be well separated when they are being cut.
- We don't want to expose the bands that are cut out later to too much excess UV.
| Lane | Sample (27 μL) | Lane | Sample (27 μL) |
|---|---|---|---|
| 1 | DNA ladder (load 10 μL) | 6 | BLANK |
| 2 | BLANK | 7 | Group 3 |
| 3 | Group 1 | 8 | BLANK |
| 4 | BLANK | 9 | DNA ladder (load 10 μL) |
| 5 | Group 2 | 10 | BLANK |
To purify your DNA from the agarose, you will use a kit from the Qiagen company. As we learned during Module 1, reagents in such commercial kits can have uninformative names and their contents are in part proprietary.
- Estimate the volume of your gel slice by weighing it.
- The easiest way to do this task is to pre-weigh an eppendorf tube (above), then weigh it again after adding the gel, and take the difference.
- What can you assume about the density of agarose and why?
- Add 3 volumes of QG for every 1 volume of agarose.
- The maximum advised volume is 550 μL. If you have a greater volume, continue in one tube for now, but first read step 6 to understand how to proceed later. Feel free to ask the teaching faculty for clarification.
- Incubate in the 50°C water bath for 10 minutes, until the agarose is completely dissolved. Every few minutes, you should remove your tube from the 50°C heat and flick or vortex it for a few seconds to help dissolve the agarose.
- Add 1 volume — original gel volume, not current solution volume — of isopropanol to the dissolved sample and pipet well to mix.
- Get one QIAquick column and one collection tube from the teaching faculty. Label the spin-column (not the collection tube!) with your team color. Gently pipet the dissolved agarose mixture onto the column. Microfuge for 60 seconds at maximum speed (approx. 16,000 rcf). The maximum capacity of the QIAquick columns is 800 uL! If you have more than 800 uL in your mixture, you will need to repeat this step using the same column.
- Discard the flow-through in a temporary waste conical tube and replace the spin-columns in their collection tubes. Add 500 μL of QG to the top of the column and spin as before.
- Discard the flow-through as before, and then add 750 μL of PE to the top of the column and incubate for 5 min at room temperature.
- Spin for 1 min at max speed.
- Discard the flow-through once more and replace the spin-column in its collection tube.
- Add nothing to the top but spin for 60 seconds more to dry the membrane.
- This step completely removes remaining ethanol.
- Meanwhile, trim the cap off of a fresh, pseudo-sterile eppendorf tube, and prepare a sticky label (in your team color) for the top: write "M2D3," your section day, and your team color. Please also add a plain sticky label to the side of the tube, so we don't lose track of whose sample is whose later on!
- Place the labeled spin-column in its matching trimmed eppendorf tube and add 30 μL of EB to the center of the membrane.
- Allow the column to sit at room temperature for one minute and then spin as before. The material that collects in the bottom of the eppendorf tubes is your purified, digested DNA.
- If you have time, join Nova (one team at a time!) to observe her measure the DNA concentration on a Nanodrop in the Niles lab just down the hall.
- Because your sample is precious and we don't have much to spare, using enough sample to get a good reading on our DU640 spectrophotometer would not be a great idea today!
- A Nanodrop can reliably evaluate nucleic acid concentrations of very small volumes.
Homework
Homework is due after Spring Break on M2D4
- During Module 1, we let you omit a methods section from your data summary… but this blissful state of affairs cannot last forever! For next time, you will take a first stab at writing up the methods for your Module 2 research article. This early draft will include just the content/procedures from Days 1-3 of lab.
- Be sure to read the Methods section guidelines at this link before you begin; doing so may save you some effort.
- As you compose your methods for the first three days (cell culture, Western blot analysis, DNA damage), do your best to think ahead about the scope of the experiment and how D1 and D2 fit into that overall context. For example, you probably want to establish your cell strains and general culture conditions just once.
- Recall that the Module 2 assignment will be done individually and with no formal revision, so it's even more important than before that you (a) complete each homework and (b) put forth your best effort – in order to get meaningful feedback that you can use later on.
- At this point, you have a lot of practice crafting figures and captions. One change between Module 1 and Module 2 is that your associated results text will be completely in narrative form, rather than partially in bullet points. Another change is that you will be describing protein and cell assays, in addition to the ubiquitous DNA gel. For M2D4, prepare a figure and caption depicting your Western blot results, as well as the associated results narrative. Here are some things to keep in mind when drafting your Results section:
- Each sub-section should begin with an overview sentence that motivates and introduces the experiment. What did you do and why did you do it?
- State the results of the experiment, minimizing any interpretation of the data (save that for the discussion!)
- Concluding sentences for each paragraph will transition to the next piece of data when possible -- stick to one topic per paragraph, but each sub-section might have a few paragraphs each.
Other details
- Your Module 1 microbiota summary revisions will be due by 5pm on Saturday after spring break. We recommend that you read your comments from Jon ASAP, and then take your time sleeping on them, prioritizing which are most important to respond to, and ultimately implementing your revision. Cramming this entire process into two days instead of two weeks is probably not wise.
- We recommend that you read the M2D4 transfection protocol and begin preparing an automated calculator in advance of class. (We will NOT collect this assignment.)
Reagent list
DNA prep
All digest reagents from New England Biolabs (NEB).
Agarose gels: 1% in TAE (see Module 1 for details)
QIAquick gel extraction kit (Qiagen)
Western Day 2
- Previously added by teaching faculty
- Odyssey blocking buffer (Licor)
- anti-Ku80 antibody
- anti-alpha tubulin antibody
- Being used today
- Donkey anti-Rabbit IR680 Antibody (Licor)
- Donkey anti-Mouse IR800 Antibody (Licor)
- TBS-T
- 50 mM Tris-Cl, to pH 7.5
- 150 mM NaCl
- Tween 20 at a final concentration of 0.1%
- Licor Odyssey System for fluorescence detection.
Next day: Journal Club I
Previous day: Begin Western protein analysis and choose system conditions