Difference between revisions of "20.109(F20):M2D4"
Noreen Lyell (Talk | contribs) (→Part 1: Prepare PF3D7_1351100 protein for SMM) |
Becky Meyer (Talk | contribs) (→Part 2: Perform SMM screen) |
||
| (13 intermediate revisions by one user not shown) | |||
| Line 18: | Line 18: | ||
===Part 1: Prepare PF3D7_1351100 protein for SMM=== | ===Part 1: Prepare PF3D7_1351100 protein for SMM=== | ||
| − | + | ||
| − | + | To prepare for the SMM experiment, dilute the concentrated PF3D7_1351100 protein to a final concentration of 0.5 μg/mL in TBS-T. | |
| − | # | + | |
| − | + | <font color = #4a9152 >'''In your laboratory notebook,'''</font color> complete the following: | |
| − | + | *Calculate the volume of PF3D7_1351100 and of TBS-T required to prepare the protein for the SMM experiment. | |
| − | + | **Concentration of concentrated PF3D7_1351100 was determined in the previous laboratory session. | |
| + | **Total volume should = 7 mL. | ||
| + | **Final concentration of PF3D7_1351100 should = 0.5 μg/mL. | ||
===Part 2: Perform SMM screen=== | ===Part 2: Perform SMM screen=== | ||
| − | #Obtain | + | |
| + | <font color = #0d368e>'''To ensure the steps included below are clear, please watch the video tutorial linked here: [[https://www.dropbox.com/s/0phy1rg1w23fs49/SMM%20Staining.mp4?dl=0 SMM Screen]]. The steps are detailed below so you can follow along!'''</font color> | ||
| + | |||
| + | #Obtain two printed SMM slides from the front laboratory bench.[[Image:Sp17 20.109 M1D4 forceps.png|thumb|250px|right|Only use forceps at the barcoded end of the slide.]] | ||
#*Only hold them at the barcoded end as you would otherwise disrupt the ligands that are printed on the surface. | #*Only hold them at the barcoded end as you would otherwise disrupt the ligands that are printed on the surface. | ||
#Record the barcode numbers of your slides in your laboratory notebook. | #Record the barcode numbers of your slides in your laboratory notebook. | ||
| − | #Carefully place each slide, barcode facing up, into a well of a 4-well chamber dish using the slide forceps.[[Image:Sp17 20.109 M1D4 pipet.png|thumb| | + | #Carefully place each slide, barcode facing up, into a well of a 4-well chamber dish using the slide forceps.[[Image:Sp17 20.109 M1D4 pipet.png|thumb|200px|right|Add all liquid to the barcoded end of the slide.]] |
#*Again, only hold the slide at the barcoded end. | #*Again, only hold the slide at the barcoded end. | ||
#Add 3 mL of your diluted protein solution to the chambers that contain your slides. | #Add 3 mL of your diluted protein solution to the chambers that contain your slides. | ||
| Line 36: | Line 41: | ||
#Gently rock the 4-well chamber dish back and forth until the slides are completely covered with the diluted protein solution. | #Gently rock the 4-well chamber dish back and forth until the slides are completely covered with the diluted protein solution. | ||
#Cover the 4-well chamber dish with aluminum foil and incubate on the rocking table for 1 hr. | #Cover the 4-well chamber dish with aluminum foil and incubate on the rocking table for 1 hr. | ||
| − | #*During all incubations it is important to keep the slides covered as the fluorescein spots used for alignment during scanning are sensitive to light.[[Image:Sp17 20.109 M1D4 liquid.png|thumb| | + | #*During all incubations it is important to keep the slides covered as the fluorescein spots used for alignment during scanning are sensitive to light.[[Image:Sp17 20.109 M1D4 liquid.png|thumb|200px|right|Pour liquid from the corner of the 4-well chamber dish.]] |
#Carefully pour the diluted protein solution from your 4-well chamber dish into a waste container by tipping the dish such that the liquid leaves the dish from the corner. | #Carefully pour the diluted protein solution from your 4-well chamber dish into a waste container by tipping the dish such that the liquid leaves the dish from the corner. | ||
#*The surface tension should keep the slides in the 4-well chamber dish; however, it is good to be careful that they do not fall into the waste container. | #*The surface tension should keep the slides in the 4-well chamber dish; however, it is good to be careful that they do not fall into the waste container. | ||
| Line 46: | Line 51: | ||
#Pour the TBS-T from the 4-well chamber dish as above into a waste container. | #Pour the TBS-T from the 4-well chamber dish as above into a waste container. | ||
#Complete Steps #8 - 10 a total of 3 times. | #Complete Steps #8 - 10 a total of 3 times. | ||
| + | #Move your slides to a fresh 4-well chamber dish to eliminate carryover from the protein and/or buffer solutions. | ||
| + | #Prepare 7 mL of anti-His antibody solution in TBS-T. | ||
| + | #*Dilute the antibody stock 1:1000 at the front laboratory bench. | ||
| + | #Add 3 mL of the diluted antibody solution to the chambers that contain your slides. | ||
| + | #*Pipet the diluted antibody solution onto the barcoded end of the slides. | ||
| + | #*Avoid generating bubbles as you add your diluted antibody solution. | ||
| + | #Gently rock the 4-well chamber dish back and forth until the slides are completely covered with the diluted antibody solution. | ||
| + | #Cover the 4-well chamber dish with aluminum foil and incubate on the rocking table for 1 hr. | ||
| + | #Carefully pour the diluted antibody solution from your 4-well chamber dish into a waste container. | ||
#Add ~3 mL of TBS (not TBS-T!) to remove excess detergent and incubate your covered 4-well chamber dish on the rocking table for 2 min, then pour liquid into a waste container. | #Add ~3 mL of TBS (not TBS-T!) to remove excess detergent and incubate your covered 4-well chamber dish on the rocking table for 2 min, then pour liquid into a waste container. | ||
| − | #Lastly, | + | #Lastly, wash the slides with dH<sub>2</sub>O. |
#*Obtain 2 reservoirs and add fresh dH<sub>2</sub>O to each. | #*Obtain 2 reservoirs and add fresh dH<sub>2</sub>O to each. | ||
#*Label the reservoirs '1' and '2'. | #*Label the reservoirs '1' and '2'. | ||
| − | #Wash each slide by removing it from the 4-well chamber dish with the slide forceps and dunking it 8 times into reservoir 1 then dunking it 8 times into reservoir 2. | + | #Wash each slide by removing it from the 4-well chamber dish with the slide forceps and dunking it 8 times into reservoir 1, then dunking it 8 times into reservoir 2. |
#Dry your slides by carefully insert your slides in the 'slide spinner' at the front laboratory bench. | #Dry your slides by carefully insert your slides in the 'slide spinner' at the front laboratory bench. | ||
#*Be mindful not to scrape the face of your slides on the slide holder. | #*Be mindful not to scrape the face of your slides on the slide holder. | ||
#*Centrifuge your slides for 30 sec. | #*Centrifuge your slides for 30 sec. | ||
#Transfer your slides into fresh 50 mL conical tubes and wrap in aluminum foil. | #Transfer your slides into fresh 50 mL conical tubes and wrap in aluminum foil. | ||
| − | # | + | #Store SMM slides at 4 °C until imaging. |
| − | + | ||
| − | ==Reagents== | + | ==Reagents list== |
*small-molecule microarray slides (a gift from Koehler Laboratory) | *small-molecule microarray slides (a gift from Koehler Laboratory) | ||
| − | *Tris-HCl buffered saline (TBS): 50 mM Tris-Hcl (pH = 7.5), 150 mM NaCl (BioRad) | + | *Tris-HCl buffered saline (TBS): 50 mM Tris-Hcl (pH = 7.5), 150 mM NaCl (from BioRad) |
| − | * | + | *TBS containing 0.1% Tween20 (TBS-T) (from BioRad) |
| + | *Alexa Fluor 647 anti-His antibody (from Qiagen) | ||
==Navigation links== | ==Navigation links== | ||
Next day: [[20.109(F20):M2D5 | Scan SMM slides to identify putative small molecule binders]] <br> | Next day: [[20.109(F20):M2D5 | Scan SMM slides to identify putative small molecule binders]] <br> | ||
Previous day: [[20.109(F20):M2D3 | Assess purity and concentration of purified protein]] | Previous day: [[20.109(F20):M2D3 | Assess purity and concentration of purified protein]] | ||
Latest revision as of 17:30, 20 August 2020
Contents
Introduction
A small molecule microarray (SMM) requires the covalent attachment of a library of small molecules to a glass slide. Our library is meant to broadly sample the drug-like chemical space (i.e. all possible chemical structures that have drug-like physical properties) and contains about 50,000 small molecules. Some libraries are much smaller, while many pharmaceutical companies possess high-throughput screening (HTS) collections of millions of compounds. Because this chemical space is very large, it’s difficult to generalize any single chemical reaction for this attachment that can be applied to all small molecules. We take a “one-size-fits-most” approach, where the glass slide is functionalized with a broadly reactive electrophile capable of reacting with nucleophiles present in most drug-like small molecules, such as alcohols or amines. Many small molecules contain multiple nucleophiles suitable for attachment. In this case, our manufacturing will result in a mixture of attachment sites. It’s important to remember that attachment to the glass slide constrains the possible orientation of the protein-small molecule interaction; some orientations are not possible because the glass slide and linker are in the way.
We start with a glass slide with exposed amines across the surface and attach a short PEG (polyethylene glycol) linker. To the end of this PEG linker, an isocyanate group is attached. Isocyanate, or R-N=C=O, is a resonant structure, and a partial positive charge is stabilized on the carbon atom. This carbon atom is electrophilic, and small molecules with nucleophiles will react here. We estimate that about 70% of drug-like small molecules are amenable to this reaction, and our library is filtered to contain only these molecules.
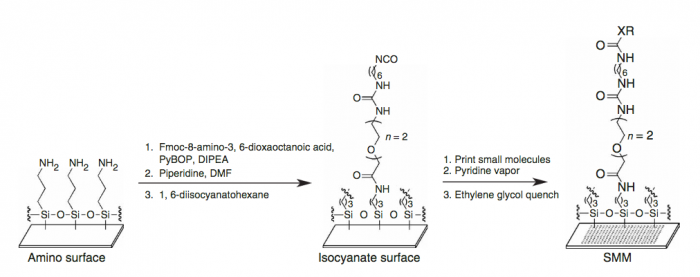
Our compound library is dissolved in DMSO and stored in 384-well plates. To dispense the compounds onto our functionalized glass slide, a robotic arm with a set of 48 metal pins is used to transfer the compounds to the glass slide. Each metal pin has a small slit in the end, and capillary action is used to precisely withdraw and dispense consistent volumes. When the pins touch the glass slide, the compound in DMSO is dispensed into a small circle of approximately 160 micron in diameter. Each pin prints one compound in two different locations on each slide, and then the pins are washed in water and DMSO. This process is repeated for each compound, resulting in our final microarray. The microarray is divided into 48 subarrays, and each subarray corresponds to one pin and contains 256 discrete spots. Within each subarray, we print a set of fluorescent compounds in the shape of an ‘X’ so that we can precisely determine where each spot is printed. After the compounds react, we quench the surface so that no electrophiles remain. This results in our final microarray; a collection of approximately 12,000 discrete spots displaying one compound each.
(Written by Rob Wilson. For more information read Bradner, J. E., McPherson, O. M., and Koehler, A. N. (2006) A method for the covalent capture and screening of diverse small molecules in a microarray format. Nature Prot. 1:2344-2352. PMID: 17406478.)
Protocols
Part 1: Prepare PF3D7_1351100 protein for SMM
To prepare for the SMM experiment, dilute the concentrated PF3D7_1351100 protein to a final concentration of 0.5 μg/mL in TBS-T.
In your laboratory notebook, complete the following:
- Calculate the volume of PF3D7_1351100 and of TBS-T required to prepare the protein for the SMM experiment.
- Concentration of concentrated PF3D7_1351100 was determined in the previous laboratory session.
- Total volume should = 7 mL.
- Final concentration of PF3D7_1351100 should = 0.5 μg/mL.
Part 2: Perform SMM screen
To ensure the steps included below are clear, please watch the video tutorial linked here: [SMM Screen]. The steps are detailed below so you can follow along!
- Obtain two printed SMM slides from the front laboratory bench.
- Only hold them at the barcoded end as you would otherwise disrupt the ligands that are printed on the surface.
- Record the barcode numbers of your slides in your laboratory notebook.
- Carefully place each slide, barcode facing up, into a well of a 4-well chamber dish using the slide forceps.
- Again, only hold the slide at the barcoded end.
- Add 3 mL of your diluted protein solution to the chambers that contain your slides.
- Pipet the diluted protein solution onto the barcoded end of the slides.
- Avoid generating bubbles as you add your diluted protein solution.
- Gently rock the 4-well chamber dish back and forth until the slides are completely covered with the diluted protein solution.
- Cover the 4-well chamber dish with aluminum foil and incubate on the rocking table for 1 hr.
- During all incubations it is important to keep the slides covered as the fluorescein spots used for alignment during scanning are sensitive to light.
- Carefully pour the diluted protein solution from your 4-well chamber dish into a waste container by tipping the dish such that the liquid leaves the dish from the corner.
- The surface tension should keep the slides in the 4-well chamber dish; however, it is good to be careful that they do not fall into the waste container.
- Add ~3 mL of TBS-T to the chambers that contain your slides.
- Remember, pipet the liquid onto the barcoded end of the slides.
- Don't 'push' the liquid across the surface of the slide with the pipet. Instead, gently rock the 4-well chamber dish back and forth to completely cover your slides.
- Incubate the slides on the rocking table for 2 min.
- Don't forget to cover the 4-well chamber dish with aluminum foil.
- Pour the TBS-T from the 4-well chamber dish as above into a waste container.
- Complete Steps #8 - 10 a total of 3 times.
- Move your slides to a fresh 4-well chamber dish to eliminate carryover from the protein and/or buffer solutions.
- Prepare 7 mL of anti-His antibody solution in TBS-T.
- Dilute the antibody stock 1:1000 at the front laboratory bench.
- Add 3 mL of the diluted antibody solution to the chambers that contain your slides.
- Pipet the diluted antibody solution onto the barcoded end of the slides.
- Avoid generating bubbles as you add your diluted antibody solution.
- Gently rock the 4-well chamber dish back and forth until the slides are completely covered with the diluted antibody solution.
- Cover the 4-well chamber dish with aluminum foil and incubate on the rocking table for 1 hr.
- Carefully pour the diluted antibody solution from your 4-well chamber dish into a waste container.
- Add ~3 mL of TBS (not TBS-T!) to remove excess detergent and incubate your covered 4-well chamber dish on the rocking table for 2 min, then pour liquid into a waste container.
- Lastly, wash the slides with dH2O.
- Obtain 2 reservoirs and add fresh dH2O to each.
- Label the reservoirs '1' and '2'.
- Wash each slide by removing it from the 4-well chamber dish with the slide forceps and dunking it 8 times into reservoir 1, then dunking it 8 times into reservoir 2.
- Dry your slides by carefully insert your slides in the 'slide spinner' at the front laboratory bench.
- Be mindful not to scrape the face of your slides on the slide holder.
- Centrifuge your slides for 30 sec.
- Transfer your slides into fresh 50 mL conical tubes and wrap in aluminum foil.
- Store SMM slides at 4 °C until imaging.
Reagents list
- small-molecule microarray slides (a gift from Koehler Laboratory)
- Tris-HCl buffered saline (TBS): 50 mM Tris-Hcl (pH = 7.5), 150 mM NaCl (from BioRad)
- TBS containing 0.1% Tween20 (TBS-T) (from BioRad)
- Alexa Fluor 647 anti-His antibody (from Qiagen)
Next day: Scan SMM slides to identify putative small molecule binders