Difference between revisions of "20.109(F20):M1D1"
Noreen Lyell (Talk | contribs) (→Part 2: Learn cell culture best practices) |
Becky Meyer (Talk | contribs) (→Part 2: Learn cell culture best practices) |
||
| (26 intermediate revisions by 2 users not shown) | |||
| Line 27: | Line 27: | ||
One major objective for this experimental module is for you to learn best practices for cell culture using correct sterile techniques. Pay close attention to the demonstration provided by the Instructor! | One major objective for this experimental module is for you to learn best practices for cell culture using correct sterile techniques. Pay close attention to the demonstration provided by the Instructor! | ||
| − | Review the following resource before you complete the tasks detailed in this exercise: | + | Review the following resource before you complete the tasks detailed in this exercise:[[20.109(F20):Guidelines_for_working_in_the_tissue_culture_facility | Guidelines for working in the tissue culture room]] |
| − | + | <font color = #0d368e>'''To ensure the steps included below are clear, please watch the video tutorial linked here: [[https://www.dropbox.com/s/q6odtv695ntih1c/Cell%20Culture.mp4?dl=0 Cell Culture]]. The steps are detailed below so you can follow along!'''</font color> | |
| − | + | ||
| − | + | ||
| − | <font color = #0d368e>'''To ensure the steps included below are clear, please watch the tutorial | + | |
'''Preparing tissue culture hood''' | '''Preparing tissue culture hood''' | ||
| Line 44: | Line 41: | ||
#*Look first at the color and clarity of the media. Fresh media is reddish-orange in color and if the media in your flask is yellow or cloudy, it could mean that the cells are overgrown, contaminated, or starved for CO<sub>2</sub>. | #*Look first at the color and clarity of the media. Fresh media is reddish-orange in color and if the media in your flask is yellow or cloudy, it could mean that the cells are overgrown, contaminated, or starved for CO<sub>2</sub>. | ||
#*Next, look at the cells using the inverted microscope. Note their shape, arrangement, and how densely the cells cover the surface of the flask. | #*Next, look at the cells using the inverted microscope. Note their shape, arrangement, and how densely the cells cover the surface of the flask. | ||
| − | #After you look at your cells, take the flask to your tissue culture hood to begin the | + | #After you look at your cells, take the flask to your tissue culture hood to begin the splitting procedure. |
| − | # | + | #With a 10 mL pipet tip, transfer the cell suspension from the T75 flask into a 10 mL conical tube. |
| − | # | + | #Carefully carry the 10 mL conical tube to the centrifuge and collect the cells at 500 rpm for 3 minutes. |
| − | # | + | #Return to the tissue culture hood and use the aspirator to remove the media from the cell pellet. |
| − | # | + | #*Attach a fresh pastuer pipet tip to the aspirator tubing before removing the media. |
| + | #*Be very careful when aspirating the media as it is easy to disturb the pellet! | ||
| + | #With a 5 mL pipet tip, add 5 mL of fresh media to the cell pellet. | ||
| + | #Resuspend the cell pellet by pipetting up-and-down with a 2 mL pipet tip. | ||
#*2 mL pipets are tricky! They fill up quickly. '''Be careful not to pull up the liquid too quickly or it will go all the way up your pipet into the pipet-aid!''' If this happens, please alert the teaching faculty rather than returning the pipet-aid to the rack. | #*2 mL pipets are tricky! They fill up quickly. '''Be careful not to pull up the liquid too quickly or it will go all the way up your pipet into the pipet-aid!''' If this happens, please alert the teaching faculty rather than returning the pipet-aid to the rack. | ||
| − | + | #Transfer 90 μL of your cell suspension from the 15 mL conical tube into a labeled eppendorf tube. | |
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | #Transfer 90 μL of your cell suspension from the 15 mL conical tube into a labeled eppendorf tube. | + | |
'''Counting cells'''<br> | '''Counting cells'''<br> | ||
| Line 68: | Line 60: | ||
#Carry the tube with your 90 μL cell suspension aliquot to the center microscope bench and add 10 μL of trypan blue cell stain. Mix by pipetting up and down. | #Carry the tube with your 90 μL cell suspension aliquot to the center microscope bench and add 10 μL of trypan blue cell stain. Mix by pipetting up and down. | ||
#Carefully pipet 10 μL of the stained cells between the hemocytometer and (weighted) glass cover slip. | #Carefully pipet 10 μL of the stained cells between the hemocytometer and (weighted) glass cover slip. | ||
| − | #Count the cells that fall within the four corner squares (with a | + | #Count the cells that fall within the four corner squares (with a 4 x 4 etched grid pattern), '''average''' (''i.e.'' divide by 4), and then multiply by 10,000 to determine the number of cells/mL. |
| − | #* | + | |
| + | <font color = #4a9152 >'''In your laboratory notebook,'''</font color> complete the following: | ||
| + | |||
| + | *Use the information provided to calculate the density of the cell culture. These were the counts of the hemocytometer corner quadrants from the video: 127, 104, 131, 122. | ||
| + | *Calculate the volume of cell suspension that contains 250,000 cells. | ||
| + | |||
| + | ===Part 3: Research MCL-5 cell line=== | ||
| − | + | Review and discuss the Abstract and Introduction from the following journal article with your laboratory partner: | |
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | Crespi ''et al.'' "[[Media:MCL-5 cell reference.pdf|A metabolically competent human cell line expressing five cDNAs encoding procarcinogenic-activating enzymes: application to mutagenicity testing.]]" ''Chemical Research in Toxicology''. (1991) 4:566-572. | |
| − | + | ||
| − | + | In this article, the researchers describe the development of the MCL-5 cell line, which was used to generate the data you will analyze in this module. | |
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | = | + | <font color = #4a9152 >'''In your laboratory notebook,'''</font color> complete the following: |
| − | + | *What is the parental cell line of the MCL-5 cells? What was the original source of the parental cells (organism, tissue, and cell type)? | |
| − | + | **Hint: the ATCC (American Type Culture Collection) is a great resource when researching cell lines! | |
| − | # | + | *Briefly, what genetic modifications were made to create the MCL-5 cell line? |
| − | + | *Why do the authors suggest that these modifications will make MCL-5 a useful model cell line for carcinogen exposure experiments? | |
| − | + | *Is the MCL-5 cell line appropriate to use in addressing the research question we are asking in this module? Why or why not? | |
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
==Reagents list== | ==Reagents list== | ||
| − | * | + | *Human β-lymphoblastoid cell line MCL-5 (a gift from the Engelward Laboratory) |
| − | * | + | *Roswell Park Memorial Institute (RPMI) medium 1640 (from Gibco), supplemented with: |
**10% fetal bovine serum (FBS) (from Atlanta Biologicals) | **10% fetal bovine serum (FBS) (from Atlanta Biologicals) | ||
| − | ** | + | **1% L-glutamine (from ThermoFisher) |
**100 U/mL of antibiotic solution, containing penicillin and streptomycin (from Gibco) | **100 U/mL of antibiotic solution, containing penicillin and streptomycin (from Gibco) | ||
| − | |||
| − | |||
*trypan blue (from VWR) | *trypan blue (from VWR) | ||
*incubator maintains 37°C, 5% CO2 and 95% relative humidity | *incubator maintains 37°C, 5% CO2 and 95% relative humidity | ||
==Navigation links== | ==Navigation links== | ||
| − | Next day: | + | Next day: [[20.109(F20):M1D2 | Prepare and treat cells for repair foci experiment]]<br> |
| + | Previous day: [[20.109(F20):Lab tour | Orientation and laboratory tour]] | ||
Latest revision as of 23:22, 1 September 2020
Contents
Introduction
In the past century, we have learned a tremendous amount by studying the behavior of mammalian cells maintained in the laboratory. Tissue culture was originally developed about 100 years ago as a method for learning about mammalian biology. The term tissue culture was coined because people were doing exactly that, extracting tissue and letting it live in a dish for a short time. Today, most tissue culture experiments are done using isolated cells rather than whole tissues. Much of what we know about cancer, heritable diseases, and the effects of the environment on human health has been derived from studies of cultured cells.
Cells that are isolated from tissue are called primary cells, because they come directly from an animal. It is very difficult to culture primary cells, largely because primary cells that are grown in culture divide only a limited number of times. This limitation on the lifespan of cultured primary cells, called the Hayflick limit, is a problem because it requires a researcher to constantly remove tissues from animals in order to complete a study. Cell isolation processes can be quite labor-intensive, and also can complicate data analysis due to inherent animal-to-animal variation. To get around the first of these problems, researchers use cells that are immortal, which means they can divide indefinitely, though some inherent cell-to-cell variation still exists in such cells.
One familiar type of immortalized cell is the cancer cell. Tumor cells continuously divide, allowing cancer to invade tissues and proliferate. Cancer cells behave the same way in culture, and under the right conditions, cells can be taken from a tumor and divide indefinitely in culture. Another type of immortalized cell is the embryonic stem cell. Embryonic stem cells are derived from an early stage embryo, and these cells are completely undifferentiated and pluripotent, which means that under the right conditions, they can become any mammalian cell type.
In this module, you will use the human β-lymphoblastoid cell line MCL-5. The MCL-5 cell line was generated by transfecting five human cDNAs that encode procarcinogen-activating enzymes into AHH-1 TK +/- human β-lymphoblastoid cells. In the exercise that you complete today, you will learn more about how this cell line is used.The art of tissue culture lies in the ability to create conditions that are similar to what a cell would experience in an animal, namely 37 °C and neutral pH. Blood nourishes the cells in an animal, and blood components are used to feed cells in culture. Serum, the cell-free (and clotting-factor free) component of blood, contains many of the factors necessary to support the growth of cells outside the animal. Consequently, serum is frequently added to tissue culture medium, although serum-free media exist and support some types of cultured cells. Furthermore, cultured mammalian cells must grow in a germ-free environment and researchers using tissue culture must be skilled in sterile technique.
Protocols
Part 1: Complete Orientation quiz
Complete the orientation quiz with your partner. Though you are working with your partner, each student should record all answers independently. If you disagree with your partner on an answer, you should write what you think is the correct answer on your quiz.
Good luck!
Part 2: Learn cell culture best practices
One major objective for this experimental module is for you to learn best practices for cell culture using correct sterile techniques. Pay close attention to the demonstration provided by the Instructor!
Review the following resource before you complete the tasks detailed in this exercise: Guidelines for working in the tissue culture room
To ensure the steps included below are clear, please watch the video tutorial linked here: [Cell Culture]. The steps are detailed below so you can follow along!
Preparing tissue culture hood
- The tissue culture hood is partly set up for you. Finish preparing your hood according to the demonstration, first bringing in any remaining supplies you will need, then obtaining the pre-warmed reagents from the water bath, and finally retrieving your cells from the 37 °C incubator.
- Be sure to spray everything (except cells) with 70% ethanol and wipe dry before moving items into the tissue culture hood!
- One of the greatest sources for tissue culture contamination is moving materials in and out of the hood because this disturbs the air flow that maintains a sterile environment inside the hood. Think about what you will need during your experiment to avoid moving your arms in and out of the hood while you are handling your cells.
Collecting cells
- Obtain one ~48 h cultures of MCL-5 cells in T75 flask from the 37 °C incubator.
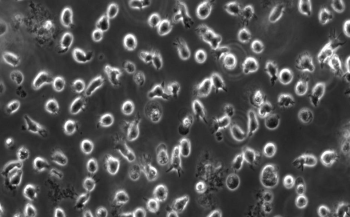
- Examine your cell cultures after you remove the flask from the incubator.
- Look first at the color and clarity of the media. Fresh media is reddish-orange in color and if the media in your flask is yellow or cloudy, it could mean that the cells are overgrown, contaminated, or starved for CO2.
- Next, look at the cells using the inverted microscope. Note their shape, arrangement, and how densely the cells cover the surface of the flask.
- After you look at your cells, take the flask to your tissue culture hood to begin the splitting procedure.
- With a 10 mL pipet tip, transfer the cell suspension from the T75 flask into a 10 mL conical tube.
- Carefully carry the 10 mL conical tube to the centrifuge and collect the cells at 500 rpm for 3 minutes.
- Return to the tissue culture hood and use the aspirator to remove the media from the cell pellet.
- Attach a fresh pastuer pipet tip to the aspirator tubing before removing the media.
- Be very careful when aspirating the media as it is easy to disturb the pellet!
- With a 5 mL pipet tip, add 5 mL of fresh media to the cell pellet.
- Resuspend the cell pellet by pipetting up-and-down with a 2 mL pipet tip.
- 2 mL pipets are tricky! They fill up quickly. Be careful not to pull up the liquid too quickly or it will go all the way up your pipet into the pipet-aid! If this happens, please alert the teaching faculty rather than returning the pipet-aid to the rack.
- Transfer 90 μL of your cell suspension from the 15 mL conical tube into a labeled eppendorf tube.
Counting cells
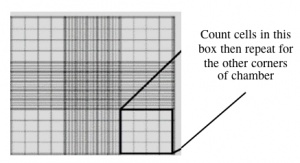
During your work in tissue culture, you will use a hemocytometer to count mammalian cells. More importantly, you will use the cell count information to determine the density of your cultures. A hemocytometer is a modified glass microscope slide that has a chamber engraved with a grid. Stained mammalian cells are loaded into the chamber, which is manufactured such that the area within the gridlines is known and the volume of the chamber is known. These features enable researchers to count the number of cells within a specific volume of liquid.
Using a hemocytometer, you can determine the density (cells per mL) of cell culture.
- Carry the tube with your 90 μL cell suspension aliquot to the center microscope bench and add 10 μL of trypan blue cell stain. Mix by pipetting up and down.
- Carefully pipet 10 μL of the stained cells between the hemocytometer and (weighted) glass cover slip.
- Count the cells that fall within the four corner squares (with a 4 x 4 etched grid pattern), average (i.e. divide by 4), and then multiply by 10,000 to determine the number of cells/mL.
In your laboratory notebook, complete the following:
- Use the information provided to calculate the density of the cell culture. These were the counts of the hemocytometer corner quadrants from the video: 127, 104, 131, 122.
- Calculate the volume of cell suspension that contains 250,000 cells.
Part 3: Research MCL-5 cell line
Review and discuss the Abstract and Introduction from the following journal article with your laboratory partner:
Crespi et al. "A metabolically competent human cell line expressing five cDNAs encoding procarcinogenic-activating enzymes: application to mutagenicity testing." Chemical Research in Toxicology. (1991) 4:566-572.
In this article, the researchers describe the development of the MCL-5 cell line, which was used to generate the data you will analyze in this module.
In your laboratory notebook, complete the following:
- What is the parental cell line of the MCL-5 cells? What was the original source of the parental cells (organism, tissue, and cell type)?
- Hint: the ATCC (American Type Culture Collection) is a great resource when researching cell lines!
- Briefly, what genetic modifications were made to create the MCL-5 cell line?
- Why do the authors suggest that these modifications will make MCL-5 a useful model cell line for carcinogen exposure experiments?
- Is the MCL-5 cell line appropriate to use in addressing the research question we are asking in this module? Why or why not?
Reagents list
- Human β-lymphoblastoid cell line MCL-5 (a gift from the Engelward Laboratory)
- Roswell Park Memorial Institute (RPMI) medium 1640 (from Gibco), supplemented with:
- 10% fetal bovine serum (FBS) (from Atlanta Biologicals)
- 1% L-glutamine (from ThermoFisher)
- 100 U/mL of antibiotic solution, containing penicillin and streptomycin (from Gibco)
- trypan blue (from VWR)
- incubator maintains 37°C, 5% CO2 and 95% relative humidity
Next day: Prepare and treat cells for repair foci experiment