Difference between revisions of "20.109(F19):Treat cells for gamma-H2AX (Day2)"
From Course Wiki
Noreen Lyell (Talk | contribs) (→Part 2: Prepare CometChip) |
Noreen Lyell (Talk | contribs) (→Reagents list) |
||
| Line 50: | Line 50: | ||
==Reagents list== | ==Reagents list== | ||
'''γH2AX''' | '''γH2AX''' | ||
| − | + | *methyl methanosulfonate (MMS) (from Sigma) | |
| + | *arsenite (As) (from Sigma) | ||
| + | *5-ethyl-2'-deoxyuridine (EdU) (from ThermoFisher Scientific) | ||
'''CometChip''' | '''CometChip''' | ||
*agar, normal melting point (from Invitrogen) | *agar, normal melting point (from Invitrogen) | ||
Revision as of 19:31, 21 August 2019
Contents
Introduction
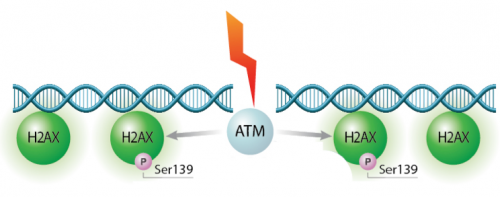
In eukaryotes, including humans, DNA is tightly wound around histone groups. H2AX is a member of the core group of histones that contributes to nucleosome formation and DNA structure. When a DNA double-strand break is introduced into the genome, the H2AX histones near the break are phosphorylated by the ATM kinase at residue Ser-139. Upon phosphorylation H2AX is referred to as gamma-H2AX. Given that only H2AX histones near the site of DNA damage are phosphorylated, γH2AX is a useful target when determining the abundance and location of double-strand breaks.
In your γH2AX assay experiment, you will assess the effects of ...
Protocols
Part 1: Treat cells for γH2AX assay
Part 2: Prepare CometChip
- Obtain a sheet of gelbond film from the laboratory bench at the front of the room. The paper is protecting the hydrophilic side of the gelbond film.
- Be sure to keep the paper associated with the gelbond film so you know which side is which.
- Also obtain a special permanent marker from the front bench (Secureline Marker II).
- If you use a marker from your drawer the ink will wash off during a later step in the CometChip assay protocol.
- Use the ruler in your team drawer and the Secureline permanent marker to draw a 4.5 x 3.5 cm rectangle near the center on the hydrophobic side of the gelbond film. Label three rows--'A', 'B', and 'C'--along the outside of the long side of the rectangle.
- Note: you are writing on what will be the bottom of the CometChip and may want to write backwards so the labels are clear when you look at the top of your CometChip.
- Prepare 20 mL of 1% normal melting point (NMP) agarose. Be careful as the agarose solution will be very hot!
- Calculate the amount of NMP agarose powder needed for a 1% w/v solution. Check your math with the teaching faculty before you continue.
- Obtain a small milk bottle from the front bench.
- Weigh out the appropriate amount of NMP agarose and add it to the milk bottle.
- Use a cylinder to measure 20 mL of 1x PBS and add it to the milk bottle with the NMP agarose powder.
- Swirl to mix.
- To melt the NMP agarose, microwave the solution for 20 seconds, swirl, then microwave for 3-second intervals until all crystals are in solutions. After each interval, remove the milk bottle and gently swirl while checking for unmelted agarose crystals. It is important that the solution does NOT boil as you will lose water to evaporation and the density of the agarose will be altered. If your solution starts to boil, immediately remove it from the microwave and gently swirl.
- When no more crystals are visible in the solution take the milk bottle to your bench.
- Obtain a small rectangle dish (labeled "scraped lid") and the CometChip 'stamp' from the front bench.
- Add 2.5 mL of the agarose solution to the small dish, then quickly place the gelbond film in the dish with the marked hydrophobic side down. Remove the paper from the film.
- Add 13 mL of the agarose solution on top of the gelbond film.
- Slowly place the CometChip stamp on top of the agarose.
- Lower the bottom left of the stamp first, then slowly allow the stamp to 'roll' into the agarose. Be sure to leave the top right corner of the small dish accessible.
- Be careful not to introduce bubbles into the agarose and work quickly as the agarose will solidify as it cools.
- Allow the agarose to solidify, undisturbed, on your bench for 30 min.
- Add ~5 mL of 1x PBS to the small dish that contains your agarose CometChip.
- Pipet in the 1x PBS using the accessible corner.
- Slowly pull from one corner of the stamp to lift it away from your CometChip in the dish.
- If the CometChip sticks to the stamp, carefully peel it off using tweezers.
- Discard the PBS in the sink.
- Remove excess agarose from the perimeter of your CometChip using a razor blade (obtain and return razor blade to front bench).
- Clean the agarose from the bottom of your CometChip (gelbond side) using a Kimwipe.
- Place your CometChip in the small dish containing 1x PBS for storage at 4 °C until next time.
- Be sure the chip is completely submerged.
- Please return the stamp to the front bench. Never wipe the stamp, as that will ruin the microposts!
Reagents list
γH2AX
- methyl methanosulfonate (MMS) (from Sigma)
- arsenite (As) (from Sigma)
- 5-ethyl-2'-deoxyuridine (EdU) (from ThermoFisher Scientific)
CometChip
- agar, normal melting point (from Invitrogen)
- phosphate buffered saline (PBS) (from VWR)
- GelBond film (from Lonza)
Next day: Complete gamma-H2AX assay and perform loading experiment