20.109(S23):M2D1
Contents
Introduction
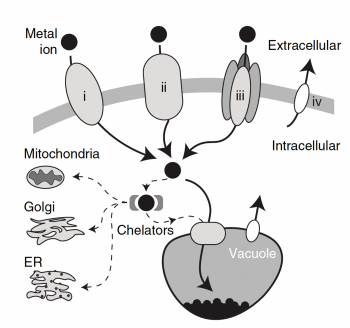
Heavy metals are typically thought of as metal elements with a relatively high density. Many of these metals are considered precious (gold, silver, platinum) or have commercial value (lead, copper, nickel). Other metals can play an essential health role in small quantities (zinc, iron, manganese). However many of these metals are toxic, even when present in parts per billion. Heavy metals can be released into the environment through a number of activities such as mining, industrial production of consumer products, or disposal of electronic waste.
In this module, you will focus on protein engineering of a metal permease in Saccharomyces cerevisiae, also known as baker's yeast, in an effort to mutate the protein such that it displays a preference for transporting the heavy metal cadmium out of the extracellular environment so that it can be collected in yeast. This type of protein engineering is an early step in creating a "bioremediation" model system. Bioremediation is an approach to cleaning up environmental pollutants by repurposing a known biological process. Typically, bioremediation utilizes microbial and fungal organisms to remove environmental contaminants as these single cell organisms can be fast growing and genetically tractable (i.e. relatively simple to genetically manipulate).
Today you examine what is known about the S.cerevisiae low-affinity iron permease, Fet4, and use that information to choose a rationally-designed mutation in an effort to increase the ability of the transporter to take up the heavy metal cadmium. To do this, you will familiarize yourself with the primary and tertiary structure of Fet4 and also study the effects of previous mutations on transporter function.
Protocols
Part 1: Review Fet4 reference article and database entry
In this module, we will attempt to alter the affinity of the S. cerevisiae low-affinity iron transporter, Fet4, to preferentially take up cadmium from the extracellular environment. We will employ a rational design approach to change specific amino acids in the Fet4 protein sequence in order to alter transporter affinity for cations. In order to guide your rational design, the lab today will encourage you to consult resources in the literature and field to determine which modifications are most promising. You will begin by examining one of the first characterizations of the Fet4 protein.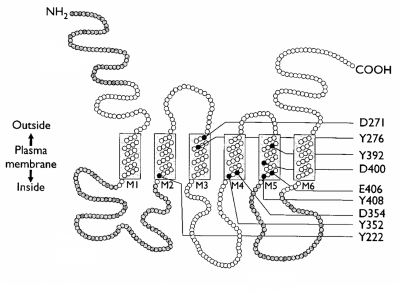
With your partner, review the information regarding the characterization of Fet4 in the paper by Dix et. al. (attached here). Specifically, you will focus on the membrane topology in Figure 1 (shown to the right) and the point mutations the authors created to alter transporter uptake of iron. These point mutations are also shown in Figure 1 and characterized in Figure 8.
In your laboratory notebook, complete the following:
- How did the authors determine their proposed topology of Fet4? What are the benefits and limitations of this approach?
- Why did the authors choose to use alanine to replace the amino acid residues they targeted?
- Which mutations had limited effect on iron uptake kinetics? Which abolished the ability of Fet4 to take up iron?
- What mutations had a moderate effect of iron uptake kinetics?
- Are there any topology commonalities shared by the different groups of mutants discussed in Table 1?
- What biological issue would explain the abolishment of iron uptake by E406A?
- Based on the information presented in this paper, on which region(s) might you focus if you want to alter Fet4 affinity for iron?
While not extensive, additional characteristics have been predicted in the InterPro database entry for Fet4. InterPro [linked here] is an aggregate database that uses models from multiple protein databases to make predictions about functional domains of proteins. To glean more predicted information about Fet4 protein structure and function, enter P40988 into the search bar. This is the designation for the S. cerevisiae Fet4 with which you will be working. Examine the different predicted features of the protein and record your observations in your lab notebook. You may also find uniprot [linked here] to be useful as well.
In your laboratory notebook, complete the following:
- How many transmembrane domains are predicted for Fet4 in this database? Does it match the predictions of Dix. et. al?
- What amino acids comprise the predicted permease domains of Fet4? In which predicted transmembrane domains are the permease domains found?
Part 2: Examine Fet4 structural elements
In the previous section you reviewed primary scientific literature to examine Fet4 topology and potentially interesting mutations in the Fet4 sequence. Now you will examine 3D representations of Fet4 topology to further examine potentially relevant amino acid residues. You will need PyMOL to complete this exercise, which can be downloaded from MIT IS&T or found on a laptop.
- The predicted structure of S. cerevisiae can be found in the AlphaFold database linked here. Please enter P40988 into the search bar to locate the organism-specific structure.
- Download the .pbd file of the predicted structure of Fet4 and open it with PyMOL.
- Begin by opening the protein sequence for the structure. To do this select Display -> Sequence.
- Click on the N-terminal methionine in the sequence and locate the corresponding residue in the structure.
- Select the C-terminal lysine and locate this residue in the structure (hint: you may need to rotate the structure to locate it).
- Refer to the proposed topology in the figure above and orient your protein so that the N-terminus and C-terminus are oriented as they appear in the figure.
- Now you can examine the broad topology of the permease to confirm the predicted topology. Remember that the Dix et. al paper used the "positive inside" rule to orient the protein at the cell surface. Now we can use predicted vacuum electrostatic interactions to confirm this prediction in a 3D structure.
- To look at overall electrostatic charges, click on "A" next to the protein name -> generate -> vacuum electrostatics -> protein contact potential
- This will show a gradient of charges with red being largely negative, blue being largely positive, and white being largely neutral.
- Determine whether or not you think the electrostatic map appears to agree with the topology predicted by Dix et. al. Are the largely negative residues located in the extracellular space?
- For reasons beyond this exercise, we can assume the neutral amino acid residues represent the transmembrane domains of the protein.
- Once you are comfortable with the overall orientation of the permease, you can examine individual residues more carefully.
- The electrostatic view can be deselected by clicking on the highlighted name "AF-P40988-F1-mod" so that you return to the original ribbon view.
- There are visualization approaches that can help match sequence and 3D structure. One is to color code regions of the protein that correspond with certain structural elements.
- To do this, select the color "C" button on the far right of the protein name -> by ss, then chose the color scheme most helpful to you.
- You can also color code individual amino acid residues to locate them in the structure by indicating the residue number and chosen color into the command space.
- For example, if you wanted to mark the tyrosine at the 301 place, you would type "color orange, resi 301".
- To show side chains, select show "S" -> side chain -> sticks. This can help you visualize functional groups.
In your laboratory notebook, complete the following:
- Based on the electrostatic map, does the overall orientation of the predicted topology match between the 3D model and published literature?
- Take screenshot(s) of the locations of the mutants from Dix et. al and note any patterns or proximity between mutations that might explain their efficacy.
Part 3: Examine data for cadmium affinity and uptake
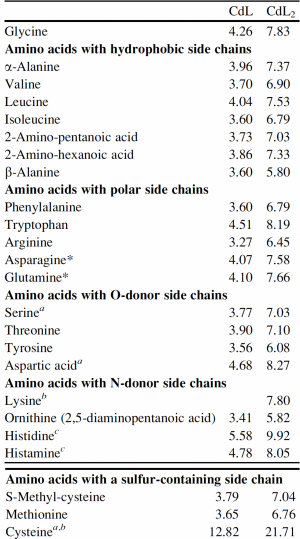
Now that you have noted relevant information regarding the Fet4 transporter, it's time to turn your attention to our metal of interest, cadmium. While there is limited information on the specific mechanism of transporter-mediated cadmium uptake into cells, previous literature has indicated that amino acid residues form complexes of differing stability with cadmium. Using the table to the right, examine the reported affinities of cadmium for different amino acid residues.In your laboratory notebook, complete the following:
- Based on the information above, which amino acids are most likely to show a preference for cadmium binding?
In your laboratory notebook, complete the following:
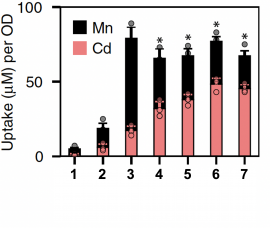
- Which mutations in SMF1 had the largest effect on cadmium uptake?
- Was a single point mutation able to alter cadmium uptake? What was the magnitude of the effect on cadmium and manganese uptake?
Part 4: Identify amino acid substitution target for Fet4_mutant
Using the information you have gathered above, you can now determine a Fet4 mutation that will potentially alter the transportation of cadmium. After you choose which amino acid you think is the best target for altering affinity, consider what amino acid you want to include instead.
In your laboratory notebook, complete the following:
- What amino acid will you target using SDM? At what position is this amino acid located in the protein sequence? What amino acid will be incorporated in its place?
- Provide the rational for your design choice.
- Why do you think the target amino acid you selected will alter affinity for cadmium?
- How do you think the amino acid substitution will alter affinity for cadmium?
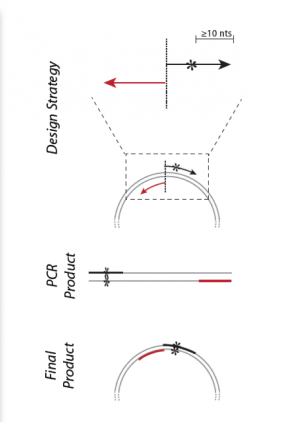
Part 5: Design primers for site-directed mutagenesis
It is not experimentally efficient, or entirely plausible, to pick out and modify a single amino acid residue in the Fet4 transporter post-translationally. Instead researchers genetically encode for amino acid substitutions by incorporating mutations in the DNA sequence. This is accomplished by making changes to the basepairs of a gene of interest that was cloned into a plasmid. Then the plasmid with the mutated gene is amplified using bacterial cells.
Primers used in SDM must meet several design criteria to ensure specificity and efficiency. Consider the following design guidelines for mutagenesis primers:
- Desired mutation (1-2 bp) must be present in the middle of the forward primer.
- Forward and reverse primers should 'face' away from the mutation and be 'back-to-back' when annealed to the template.
- Primers should be 25-45 bp long.
- G/C content of > 40% is desired.
- Both primers should terminate in at least one G or C base.
- Melting temperature should exceed 78°C, according to:
- Tm = 81.5 + 0.41 (%GC) – 675/N - %mismatch
- where N is primer length and the two percentages should be integers
To demonstrate primer design, the illustration below uses S101L, which is an uninteresting mutation but a helpful example:
Residue 101 of the protein calmodulin is serine, encoded by the AGC codon.
361 (5') GAG GAA ATC CGA GAA GCA TTC CGT GTT TTT GAC AAG GAT GGG AAC GGC TAC ATC AGC GCT (3')
381 (5') GCT CAG TTA CGT CAC GTC ATG ACA AAC CTC GGG GAG AAG TTA ACA GAT GAA GAA GTT GAT (3')
To change from serine to leucine, one might choose TTA, TTG, or CTN (wherer N = T, A, G, or C). Because CTC requires only two mutations (rather than three as for the other options), we choose this codon.
Now we must keep >10 bp of sequence on each side in a way that meets all our requirements. To quickly find G/C content and see secondary structures, look at the IDT website. (Note that the Tm listed at this site is not one that is relevant for mutagenesis.)
Ultimately, your forward primer might look like the following, which has a Tm of almost 81°C, and a G/C content of ~58%.
5’ GG AAC GGC TAC ATC CTC GCT GCT CAG TTA CGT CAC G 3'
The reverse primer is the inverse complement of a sequence just preceding the forward primer in the IPC gene. The forward and reverse primers are set up back-to-back.
Luckily, online tools are available to assist with SDM primer design. Today you will use NEBaseChanger (provided by NEB) to design your mutagenic primers.
- Go to the NEBaseChanger site and click 'Please enter a new sequence to begin.'
- A new window will open.
- Download the WT Fet4 sequence here.
- Copy the sequence from this file into the NEB window.
- Confirm that the 'Substitution' option is selected.
- Highlight the basepairs you want to mutate using by scrolling through the sequence, or you can search the sequence by typing the basepairs into the 'Find' box.
- Type the new DNA sequence (the basepair(s) you want your forward mutagenic primer to incorporate into the Fet4 sequence) in the 'Desired Sequence' box.
- Under the Result header, a diagram showing where your primers will anneal is provided.
- Under the Required Primers header, the sequences for your forward primer and reverse primer are shown with the characteristics for each.
In your laboratory notebook, complete the following:
- Include a screen capture of the information provided in the Result and Required Primers sections.
- Use the guidelines provided above to examine the mutagenesis primers designed by NEBaseChanger. Do the primers meet the design criteria?
Copy your forward and reverse primer sequence and upload them to the Class Data page on the wiki before you leave.
- These primers must be ordered tonight to arrive in time for your next experiment.
Next day: Perform site-directed mutagenesis