20.109(S20):Examine TDP43 binders for chemical features (Day7)
Contents
Introduction
Proteins and small molecules interact in a process referred to as molecular recognition. In molecular recognition, the non-covalent complexes that form are defined by two characteristics: specificity and affinity.
- Specificity distinguishes a specific binding partner from the milieu of potential binding partners in complex environments.
- Affinity dictates the likelihood of binding based on the concentration of a specific binding partner in a milieu of potential binding partners such that high affinity partners at low concentrations are not outcompeted by low affinity partners and high concentrations.
In cells, proteins are critical macromolecules that perform numerous roles related to structure, mechanics, metabolism, and signaling. In these roles, proteins do not work in a vacuum; rather the biological roles of proteins are dependent on direct physical interactions with other molecules. Though our focus is on small molecule binders, proteins also interact with other proteins, nucleic acids, oxygen, and metals. In all cases, the interactions are characterized by protein-ligand-solvent binding kinetics.
Though beyond the scope of this module, protein-ligand-solvent binding kinetics are defined as a thermodynamic system composed of solute (proteins and binders) and solvent (liquid that contains the proteins and binders). In that protein-small molecule complexes result in heat transfer, the driving forces that promote these interactions are due to energy exchanges that are characterized by Gibbs free energy (ΔG). ΔG measures the capacity of a thermodynamic system to do maximum or reversible work at constant temperature and pressure. When at equilibrium with constant temperature and pressure, protein-small molecule binding occurs when the change in ΔG is negative. The magnitude of ΔG provides insight into the stability of a protein-small molecule complex.
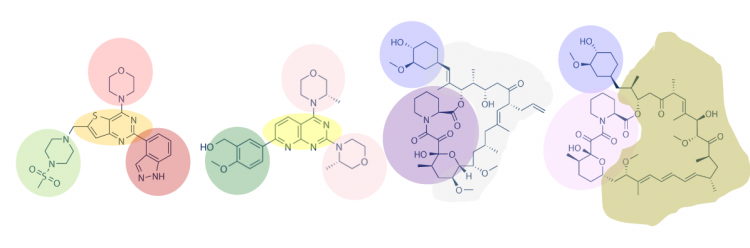
Another method for assessing protein-small molecule binding is to visually inspect known small molecule binders for common features / structures. Though you may not realize it, you did this on M1D2! Remember the information regarding lactose and IPTG? In both of these examples, the molecules share a feature that enables binding to the same target (LacI for lactose and IPTG). Your goal for today is to carefully examine the hits identified by the class and identify any common features / structures. As in the image below, it is possible that multiple features will be present within the same small molecule.
Protocols
Part 1: Visually evaluate chemical structures of positive hits
With your partner, review the hits identified in the SMM. It may be easier to copy / paste the small molecule images into a powerpoint file so you can readily see all of the structures. Also, it may be helpful to use a color-coding system (like the one in the image provided in the Introduction) to highlight features / structures that are common to the TDP43-RRM12 binders.
Discuss the following questions with your partner and record your thoughts in your laboratory notebook:
- How many features did you identify that are present in two or more of the TDP43-RRM12 ligand binders? Are there more or less than you expected?
- Is there a feature present in all of the identified binders? What might this suggest about the binding site(s) and / or binding ability of TDP43-RRM12?
- Can you assign the identified binders to sub-groups based on the common features that are present?
Part 2: Develop future works and implications section
Now that you know more about all the hits identified by the 20.109 class, refine the Future Work and Implications section you completed for homework.
Use the following questions to craft the bulleted paragraphs that will be your Future Work and Implications:
- What would you do differently to improve your experimental design?
- Consider the steps that that did work as expected and how you would improve or change the procedure for 'better' results.
- What would you do next to bolster your findings?
- Include experiments that will provide further insight on the hits you discovered in your screen.
- Think back to the in-class assignment from M1D5.
- How does your research fill the knowledge gap you introduced in the Abstract and Background sections?
- Include what is now known because of the work you completed.
- How does your research advance the field?
- Consider the 'big picture' you introduced in the Abstract and Background sections.
Next day: Prepare cells for RNA purification and practice RNA-seq data analysis methods