Difference between revisions of "Understanding the lock-in amplifier"
Steven Nagle (Talk | contribs) (→Lock-in signal detection) |
(→Lock-in signal detection) |
||
| Line 5: | Line 5: | ||
==Lock-in signal detection== | ==Lock-in signal detection== | ||
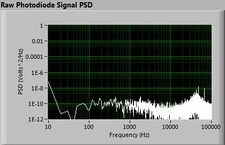
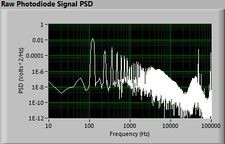
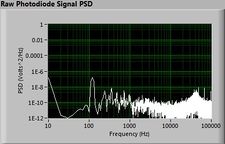
| − | + | The plots below show typical power spectral density measurements of noise in the 20.309 lab — a mix of optical and electronic noise. Fluorescent lighting creates a very strong technical noise at 120 Hz and harmonics. The lock-in technique used in the DNA lab involves modulating the blue light that excites the fluorescent dye in the sample to move the signal spectrum from the riotous low frequency realm to a calmer range of frequencies. Lock-in signal detection provides some immunity to the very noisy lab environment. | |
| + | |||
| + | Noise in lab the may look different than in previous semesters, so it may be useful to measure the noise spectrum before settling on a modulation frequency for your lock-in amplifier. | ||
{| | {| | ||
| Line 12: | Line 14: | ||
| | | | ||
|- | |- | ||
| − | | [[Image:Electrical_Background.jpg|225 px|thumb|center | + | | [[Image:Electrical_Background.jpg|225 px|thumb|center]] |
| − | | [[Image:Light_Electrical_Combined_Background.jpg|225 px|thumb|center | + | | [[Image:Light_Electrical_Combined_Background.jpg|225 px|thumb|center]] |
| − | | [[Image:Instrument_Light_Electrical_Combined_Background.jpg|225 px|thumb|center | + | | [[Image:Instrument_Light_Electrical_Combined_Background.jpg|225 px|thumb|center]] |
|- | |- | ||
|| | || | ||
Revision as of 00:37, 21 April 2015
Lock-in signal detection
The plots below show typical power spectral density measurements of noise in the 20.309 lab — a mix of optical and electronic noise. Fluorescent lighting creates a very strong technical noise at 120 Hz and harmonics. The lock-in technique used in the DNA lab involves modulating the blue light that excites the fluorescent dye in the sample to move the signal spectrum from the riotous low frequency realm to a calmer range of frequencies. Lock-in signal detection provides some immunity to the very noisy lab environment.
Noise in lab the may look different than in previous semesters, so it may be useful to measure the noise spectrum before settling on a modulation frequency for your lock-in amplifier.
| |
||
| |
|
|
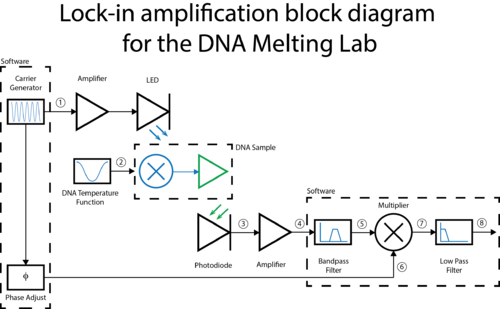
A block diagram of the lock-in scheme is shown below. Implementing lock-in detection requires the ability to modulate the LED output with a carrier frequency and requires signal processing (in software in our case) to recover the non-modulated signal. In your case the non-modulated signal is the fraction of dsDNA versus time. To support these functions, the LED circuit must be modified to include a feedback brightness controller. In addition, the photodiode amplifier must be modified to accommodate the change in signal frequency range. In the control software, you will need to choose the LED modulation frequency as well as all filter frequencies to support your design.
References