|
|
| Line 1: |
Line 1: |
| − | Back to [[QRT-PCR]]
| + | [[File:Pcr-setup.jpg|center|400px]] |
| | + | <big><center>QRT-PCR: 20.345 Spring 2011</center></big> |
| | | | |
| − | Looking for [[Spring_11:QRT-PCR_weekly_reports|weekly reports?]]
| + | ==Goal== |
| | | | |
| − | == To-Do ==
| + | The ultimate goal of this project is to create an inexpensive QRT-PCR kit for teaching laboratories at the undergraduate, or even high school, level. At the beginning of term, I had hoped to have a functional QRT-PCR machine by this time. Part way through term, it became clear that this would not happen, so I lowered my sights to traditional PCR alone. Unfortunately, even more setbacks occurred, and not even this early milestone was achieved. |
| − | Last updated 23:01, 5 May 2011 (EDT)
| + | |
| | | | |
| − | * fine-tune Tlid involvement in the model (fix it so it doesn't affect whole graph)
| + | ==Progress== |
| − | * reduce lid heater current so it doesn't melt the tube
| + | |
| | | | |
| − | == Weekly "goals" ==
| + | Some progress, however, has been made, which should be of some use to Steven, or future students. |
| − | as of 15:03, 4 April 2011 (EDT)
| + | |
| − | * 3/25: Optics finished. Code started.
| + | |
| − | * 4/1: Heated lid finished.
| + | |
| − | * 4/8: No really, heated lid finished. Updated heat transfer function. Kinda know LabView.
| + | |
| − | * 4/15: Successful PCR.
| + | |
| − | * 4/22: Optics are ready for QRT-PCR!
| + | |
| − | * 4/29: Successful QRT-PCR from the side.
| + | |
| − | * 5/6: QRT-PCR from the top. Now which one was better?
| + | |
| | | | |
| − | == Notes == | + | ===Optics=== |
| | + | At the start of term, I worked to increase the signal-to-noise ratio of the optics. After constructing and investigating several model systems of a green LED and neutral density filters, I determined that the autofluorescence of the fiber optic was producing a prohibitive amount of noise. The replacement fiber optic that was acquired also exhibited autofluorescence, so it seems that we will not be able to use a fiber optic in our design. |
| | | | |
| − | === 5/8/11 === | + | ===Electronics=== |
| − | Posted 14:27, 17 May 2011 (EDT)
| + | A bug was discovered in the H-bridge circuit: its dynamic range is now limited to 1-5 V, instead of 0-10 V. Not even a replacement H-bridge solved the problem, so the issue is somewhere beyond the basic electrical connections. |
| | | | |
| − | Ran a PCR. Used parameters as noted on 5/5/11
| + | ===Labview VI=== |
| | + | Version 2.712 was updated to calculate the temperature properly from the thermistors. When thermal coupling between the block and the lid was a problem, a Tlid gain term was added to the model temperature to account for the extra power coming from the lid. When this part of the code was no longer needed, it was removed. |
| | | | |
| − | Commercial machine: program NK1:
| + | ===Heated Lid=== |
| − | * 95C for 30 sec
| + | [[File:Heated_lid.png|right|500px]] |
| − | * repeat x 30:
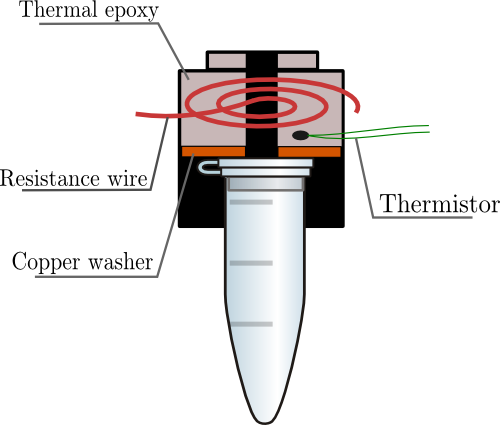
| + | A heated lid, to reduce condensation and rescue the efficacy of the PCR machine, was designed, constructed, and implemented. |
| − | ** 95C for 30 sec
| + | |
| − | ** 55C for 1 min
| + | |
| − | ** 72C for 10 sec
| + | |
| − | * 4C for infinity
| + | |
| | | | |
| − | 345 machine: 30 cycles
| + | The lid design (see right) consists of 29-gauge resistance wire, thermal epoxy, a copper wire, and a thermistor to measure the temperature. All of these are encased by a half-inch lens tube. 1.03 A of current through the resistance wire generates about 100C of heat, which is transferred to the tube lid via the thermal epoxy and the copper washer. |
| − | * 94C for 3.8 min
| + | |
| − | * repeat x 30:
| + | |
| − | ** 95C for 100 sec
| + | |
| − | ** 54C for 65 sec
| + | |
| − | ** 72C for 70 sec
| + | |
| − | * 4C for infinity.
| + | |
| | | | |
| − | began commercial at 3:05; 345 machine at 3:17
| + | 29-gauge wire was chosen to minimize the current needed to heat the wire, while still being thick enough to work with. The thermal epoxy ensures that the resistance wire does not make contact with itself, which could cause dangerously high resistance, and therefore heat, levels. |
| | | | |
| − | Observations:
| |
| − | * '''Commerical''': Tube I used was too tall, and so was misshapen upon removal. It also took longer than expected, for some reason, and was halted early.
| |
| − | * '''345''': Cap of tube was melted a bit. Was left at 4C for about an hour, which may have caused the condensation on the heating block. Ended earlier than expected - what happened?
| |
| | | | |
| − | Checked product on a gel:
| + | ==What Worked, or Not== |
| | + | As could be inferred from the above, not everything I tried this semester worked as I expected. |
| | | | |
| − | | + | To ensure contact between the lid and its heater, pressure must be applied to the lid heater. To provide this pressure, my first design involved screwing the lid heater onto the heating block. Unfortunately, this created a great deal of thermal coupling – so much so, that to appropriately model the sample temperature, I had to introduce into the Labview VI a gain term proportional to the |
| − | === 5/7/11 ===
| + | |
| − | Prepped to run PCR.
| + | |
| − | | + | |
| − | | + | |
| − | === 5/5/11 ===
| + | |
| − | Posted 23:01, 5 May 2011 (EDT)
| + | |
| − | | + | |
| − | Added a gain term to the temperature model, that kinda factors in T_lid. Now the model is a lot better; within 1-2 degrees! Which means we're ready to do a PCR next time. yay.
| + | |
| − | | + | |
| − | Parameters that result in a good model:
| + | |
| − | * Gain=0.15
| + | |
| − | * I_lid = 1.95
| + | |
| − | * K_p = 1.2
| + | |
| − | * K_i = K_d = 0
| + | |
| − | * a_zo = 11.1
| + | |
| − | * a_z1 = -9.9
| + | |
| − | * b_z = 1
| + | |
| − | | + | |
| − | === 5/1/11 ===
| + | |
| − | Posted 16:46, 1 May 2011 (EDT)
| + | |
| − | | + | |
| − | Yesterday I made sure all the thermistors were working.
| + | |
| − | Today I made sure the resistance wire was working.
| + | |
| − | | + | |
| − | Temperatures are correct in v2.712. Only the Lid temperature (which I just added) is correct in v2.52, which I need to take the frequency data. Will seek further advice from Steven tomorrow.
| + | |
| − | | + | |
| − | I am also not sure what exactly we want from our lid heater.
| + | |
| − | | + | |
| − | === 4/27/11 ===
| + | |
| − | Posted 17:59, 27 April 2011 (EDT)
| + | |
| − | | + | |
| − | FUN FACT: you can strip a thermistor wire using a razor.
| + | |
| − | | + | |
| − | Cleaned out the lid so it can be used. Then, I soldered the Lid Heater Thermistor and Sample Thermistor. I also fixed the resistance wire that broke. The lid is not hooked up, but it is ready. I wish I had been able to work faster and do more, but... I'll be back tomorrow.
| + | |
| − | | + | |
| − | | + | |
| − | === 4/26/11 ===
| + | |
| − | Posted 17:59, 27 April 2011 (EDT)
| + | |
| − | | + | |
| − | Drilled a hole in the lid for the Lid Heater Thermistor. Poured epoxy,
| + | |
| − | which is now curing. I had planned to put a washer on top of the epoxy
| + | |
| − | to keep it flat, but it wouldn't fit in there (thanks to the slight
| + | |
| − | tilt of the rod). So we'll hope for the best, and use RTV to flatten
| + | |
| − | it if all else fails.
| + | |
| − | | + | |
| − | | + | |
| − | === 4/20/11 ===
| + | |
| − | Posted 19:31, 20 April 2011 (EDT)
| + | |
| − | | + | |
| − | OK heated lid is pretty close to being actually usable. Maybe. There are holes in the sides of the cube so we can hold the resistances wires straight. This is good, because otherwise the wires melt the plastic.
| + | |
| − | | + | |
| − | The lid screws on to the base well, if you use the cut-off lens tube.
| + | |
| − | | + | |
| − | The copper wire is kept flat thanks to some RTV. It's rated for up to 150C.
| + | |
| − | | + | |
| − | Problems:
| + | |
| − | * lid does not fit inside cube thing. lid is too tall. For now this has been fixed with some black cardboardy stuff.
| + | |
| − | * I broke the sample thermistor. Whoops. :(
| + | |
| − | * the hole in the thermal epoxy is not quite centered, so you don't see as much of the sample as you could from the top. Eventually I should make a new lid to fix this....
| + | |
| − | | + | |
| − | === 4/6/11 ===
| + | |
| − | Posted 17:13, 6 April 2011 (EDT)
| + | |
| − | | + | |
| − | Learned some more about LabView.....
| + | |
| − | | + | |
| − | I've constructed the heated lid. Now I just need to hook it up electrically. Which I can't do...without some guidance, since you gotta use something called acid flux with the NiChrome. okay.
| + | |
| − | | + | |
| − | The heater in the lid is a little too tall, so that the lid doesn't quite fit under the cap. Probably smaller-diameter washers could fix this. But for now, it doesn't need to be fixed.
| + | |
| − | | + | |
| − | I still don't know what the 2.712 TEC cooling problem is. I do know that it is not:
| + | |
| − | * the PWM controller.
| + | |
| − | * the Kp, Ki, or Kd values.
| + | |
| − | | + | |
| − | My next guess is that it has to do with this weird equation, which appears to convert temperature to volts? what? Yeah, I need to talk to Steven.
| + | |
| − | | + | |
| − | I currently need to talk to Steven about:
| + | |
| − | * soldering/wiring the NiChrome wire
| + | |
| − | * TEC cooling problem
| + | |
| − | | + | |
| − | === 4/4/11 ===
| + | |
| − | Posted 17:13, 4 April 2011 (EDT)
| + | |
| − | | + | |
| − | I now know the reason for the background signal. The fiber optic cable is auto-fluorescing. BOO. It works okay for now (there is a small, but extant difference between H20 and SYBR+dsDNA), but in order to do any serious testing, I'll need to change the design. Or get a new fiber optic
| + | |
| − | | + | |
| − | It might, in fact, be time to get rid of the fiber optic all together. But I will also look around for fiber optics that really, really do not fluoresce.
| + | |
| − | | + | |
| − | Came up with a preliminary design for heated lid. Will verify with Steven.
| + | |
| − | | + | |
| − | v2.712 now uses thermistor equations instead of RTD. The correct temperature is produced. Hooray! However, The modeled temperature is quite wrong. More problematically, the TEC doesn't cool in this version (though it does in 2.52). This is probably due to the settings in 2.712 (i.e., it's comparing against the modeled temp instead of the real sample temp), as opposed to fundamental issues with the code. But I will talk to Steven about it.
| + | |
| − | | + | |
| − | === 4/1/11 ===
| + | |
| − | Posted 15:50, 1 April 2011 (EDT)
| + | |
| − | | + | |
| − | Officially ordered 29-gauge wire.
| + | |
| − | | + | |
| − | The optics *do* detect a difference between water and SYBR dsDNA, but it's a fairly small difference. I think this small difference is due to the HUGE amount of background signal at the carrier frequency. Why is this happening? My current hypothesis is that the dichroic mirror is directly reflecting some light from the blue LED into the photodiode... but with the filters in place, this shouldn't be happening!
| + | |
| − | | + | |
| − | Question: how good are the filters we're using? Could they still be letting some other light through? (maybe we need another blue filter in front of the blue LED).
| + | |
| − | | + | |
| − | Steven went over with me some of the different existing versions of the LabView code. I will be working with versions 2.700 and later. Not all of them work. I will take notes on this; take a look at [[QRT-PCR:LabView-versions]].
| + | |
| − | | + | |
| − | === 3/28/11 ===
| + | |
| − | Turns out my calculations were all messed up before! With corrected calculations, and the data from [www.heatersplus.com], I decided on 29-gauge resistance wire for our heated lid.
| + | |
| − | | + | |
| − | === 3/22/11 ===
| + | |
| − | Getting the temperature calculation right earned me a level-up... meaning I got to graduate to the Current Version of the Code. Got a brief intro to it. It's pretty complicated.
| + | |
| − | | + | |
| − | === 3/20/11 ===
| + | |
| − | Based on model system (blue LED + ND filter), I don't think gain is high enough. Increased gain of transimpedance amp from 2.5e4 to 2.5e5.
| + | |
| − | Well, it didn't help that much. So it's back the way it was. In any case, we get a significant signal (~0.25 units amplitude) with the model system. Huzzah!
| + | |
| − | | + | |
| − | After much wrestling with simple math, the temperature sensing is largely working! yay
| + | |
| − | | + | |
| − | === 3/18/11 ===
| + | |
| − | Optical setup appears to be working.
| + | |
| − | | + | |
| − | === 3/16/11 ===
| + | |
| − | Added dichroic mirror.
| + | |
| − | | + | |
| − | Question: how can I verify the optical setup? it seems to respond to driving signal, not fluorescence.
| + | |
| − | | + | |
| − | TODO: put away 25mm lens
| + | |
The ultimate goal of this project is to create an inexpensive QRT-PCR kit for teaching laboratories at the undergraduate, or even high school, level. At the beginning of term, I had hoped to have a functional QRT-PCR machine by this time. Part way through term, it became clear that this would not happen, so I lowered my sights to traditional PCR alone. Unfortunately, even more setbacks occurred, and not even this early milestone was achieved.
Some progress, however, has been made, which should be of some use to Steven, or future students.
At the start of term, I worked to increase the signal-to-noise ratio of the optics. After constructing and investigating several model systems of a green LED and neutral density filters, I determined that the autofluorescence of the fiber optic was producing a prohibitive amount of noise. The replacement fiber optic that was acquired also exhibited autofluorescence, so it seems that we will not be able to use a fiber optic in our design.
A bug was discovered in the H-bridge circuit: its dynamic range is now limited to 1-5 V, instead of 0-10 V. Not even a replacement H-bridge solved the problem, so the issue is somewhere beyond the basic electrical connections.
Version 2.712 was updated to calculate the temperature properly from the thermistors. When thermal coupling between the block and the lid was a problem, a Tlid gain term was added to the model temperature to account for the extra power coming from the lid. When this part of the code was no longer needed, it was removed.
A heated lid, to reduce condensation and rescue the efficacy of the PCR machine, was designed, constructed, and implemented.
The lid design (see right) consists of 29-gauge resistance wire, thermal epoxy, a copper wire, and a thermistor to measure the temperature. All of these are encased by a half-inch lens tube. 1.03 A of current through the resistance wire generates about 100C of heat, which is transferred to the tube lid via the thermal epoxy and the copper washer.
29-gauge wire was chosen to minimize the current needed to heat the wire, while still being thick enough to work with. The thermal epoxy ensures that the resistance wire does not make contact with itself, which could cause dangerously high resistance, and therefore heat, levels.
As could be inferred from the above, not everything I tried this semester worked as I expected.
To ensure contact between the lid and its heater, pressure must be applied to the lid heater. To provide this pressure, my first design involved screwing the lid heater onto the heating block. Unfortunately, this created a great deal of thermal coupling – so much so, that to appropriately model the sample temperature, I had to introduce into the Labview VI a gain term proportional to the