Difference between revisions of "Spring 11:Chemotaxis Assay"
| Line 1: | Line 1: | ||
| + | [[File:System 1.JPG|center|thumb|600px]] | ||
| + | |||
[[File:Microinjector_Seymour.jpg|center|thumb|600px]] | [[File:Microinjector_Seymour.jpg|center|thumb|600px]] | ||
| + | |||
== Introduction == | == Introduction == | ||
For my project I designed and developed an automated microfluidic system using Seymour et al’s microinjector microfluidic device to provide a high through-put method of acquiring bacterial chemotaxis parameters. The microinjector device was chosen for its simple design, diverse application to both fluid-flow and flow-free approaches, and for its ability to measure individual cell kinetics. The development of a faster and systematic device is especially useful in the research of marine microbes’ response to settling dissolved organic matter. | For my project I designed and developed an automated microfluidic system using Seymour et al’s microinjector microfluidic device to provide a high through-put method of acquiring bacterial chemotaxis parameters. The microinjector device was chosen for its simple design, diverse application to both fluid-flow and flow-free approaches, and for its ability to measure individual cell kinetics. The development of a faster and systematic device is especially useful in the research of marine microbes’ response to settling dissolved organic matter. | ||
| − | == Objective == | + | === Objective === |
Further develop Stocker’s microfluidic system that will allow researchers to run chemotaxis assays more efficiently for high though-put assays. The main improvement to Stocker's system will be the use of pneumatic pressures instead of syringe fluid pressures to control fluid flow to set up the chemoatractant and microorganism bands. The pneumatic pressure will be computer controlled to to allow precise and reproducible experiments as well as increased experimental throughput. | Further develop Stocker’s microfluidic system that will allow researchers to run chemotaxis assays more efficiently for high though-put assays. The main improvement to Stocker's system will be the use of pneumatic pressures instead of syringe fluid pressures to control fluid flow to set up the chemoatractant and microorganism bands. The pneumatic pressure will be computer controlled to to allow precise and reproducible experiments as well as increased experimental throughput. | ||
| Line 10: | Line 13: | ||
* [add files here, ask steve] | * [add files here, ask steve] | ||
| − | == Readings and Resources == | + | === Readings and Resources === |
* [add files and links here, ask steve] | * [add files and links here, ask steve] | ||
| + | |||
| + | === Materials and budget === | ||
| + | [[File:Budget.jpg|center|thumbnail|600px]] | ||
== Microinjector Microfluidic Device == | == Microinjector Microfluidic Device == | ||
| Line 25: | Line 31: | ||
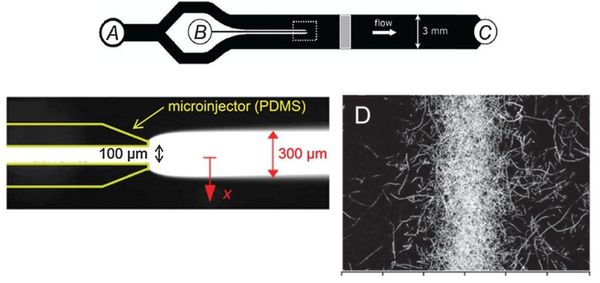
[[File: Microinjector_w-subpic.jpg|center|thumb|600px|'''Figure :Microinjector microfluidic device (Seymour 2009).''' The device has two inlets; one for a chemoeffector (inlet B) and one for a bacterial or microorganism suspension (inlet A). Bacteria and chemoeffector band created by microinjector are flowed to setup initial gradient. (B) The band width of the chemoeffector band can be adjusted by changing the flow rates into inlets A & B.]] | [[File: Microinjector_w-subpic.jpg|center|thumb|600px|'''Figure :Microinjector microfluidic device (Seymour 2009).''' The device has two inlets; one for a chemoeffector (inlet B) and one for a bacterial or microorganism suspension (inlet A). Bacteria and chemoeffector band created by microinjector are flowed to setup initial gradient. (B) The band width of the chemoeffector band can be adjusted by changing the flow rates into inlets A & B.]] | ||
| + | |||
| + | ===Dimmension=== | ||
| + | * Microinjector Channel | ||
| + | ** width: 100μm | ||
| + | ** depth: 50μm | ||
| + | ** length: | ||
| + | * Main Channel | ||
| + | ** width: 3mm | ||
| + | ** depth: 50μm | ||
| + | ** length: | ||
== Microscope == | == Microscope == | ||
| Line 31: | Line 47: | ||
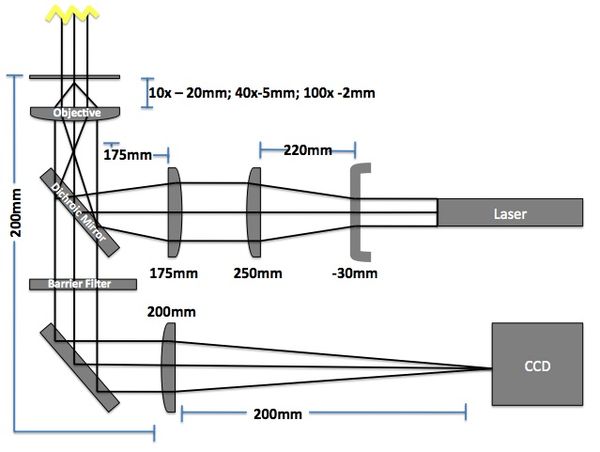
[[File:BF_Fluor_Microscope_Design.jpg|center|thumb|600px|'''Figure 3: Bright field and Fluorescent Microscope Block Diagram''']] | [[File:BF_Fluor_Microscope_Design.jpg|center|thumb|600px|'''Figure 3: Bright field and Fluorescent Microscope Block Diagram''']] | ||
| − | * Camera ([http://www.alliedvisiontec.com/fileadmin/content/PDF/Products/Technical_Manual/Manta/Manta_TechMan_V3.0.0_en.pdf Allied Manta G032B]): 7. | + | * Camera ([http://www.alliedvisiontec.com/fileadmin/content/PDF/Products/Technical_Manual/Manta/Manta_TechMan_V3.0.0_en.pdf Allied Manta G032B]): 7.4μm sqr pixels, 656 x 492 pixels |
| − | * Field of View: | + | * Field of View: 484μm x 366μm (10x objective w/200mm lens => 10x magnification) |
| + | * Red LED illumination | ||
== Pneumatic System Design == | == Pneumatic System Design == | ||
| − | The pneumatic system is composed of manual pressure regulators | + | The pneumatic system is composed of manual and electro forward pressure regulators, solenoid actuators, pneumatic tubing, and circuitry to |
=== Basic Design === | === Basic Design === | ||
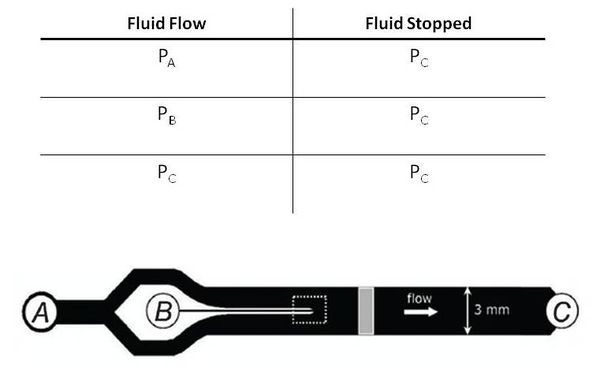
| − | The fundamental principle behind this system is that changes in pressures causes fluid flow and when the pressure equalize to the same 'ground' pressure, fluid flow should stop. By this approach | + | The fundamental principle behind this system is that changes in pressures causes fluid flow and when the pressure equalize to the same 'ground' pressure, fluid flow should stop. By this approach one is able to easily control fluid flow of the entire channel and the flow rates into inlets A and B to control the width of the chemoeffector band, dependent on the ratio of P_A and P_B. Solenoid actuators, controlled by LabView, to switch from the 'flow' pressure state to the 'stopped' state. |
[[File:Systemized_Chemotaxis_Assay_Presentation_1.jpg|center|thumb|600px|'''Figure 4: Pressure states of fluid flow]] | [[File:Systemized_Chemotaxis_Assay_Presentation_1.jpg|center|thumb|600px|'''Figure 4: Pressure states of fluid flow]] | ||
| + | |||
| + | |||
=== Circuit Element Design === | === Circuit Element Design === | ||
Revision as of 03:36, 17 May 2011
Contents
Introduction
For my project I designed and developed an automated microfluidic system using Seymour et al’s microinjector microfluidic device to provide a high through-put method of acquiring bacterial chemotaxis parameters. The microinjector device was chosen for its simple design, diverse application to both fluid-flow and flow-free approaches, and for its ability to measure individual cell kinetics. The development of a faster and systematic device is especially useful in the research of marine microbes’ response to settling dissolved organic matter.
Objective
Further develop Stocker’s microfluidic system that will allow researchers to run chemotaxis assays more efficiently for high though-put assays. The main improvement to Stocker's system will be the use of pneumatic pressures instead of syringe fluid pressures to control fluid flow to set up the chemoatractant and microorganism bands. The pneumatic pressure will be computer controlled to to allow precise and reproducible experiments as well as increased experimental throughput.
Spring 20.345 Project Assignments
- [add files here, ask steve]
Readings and Resources
- [add files and links here, ask steve]
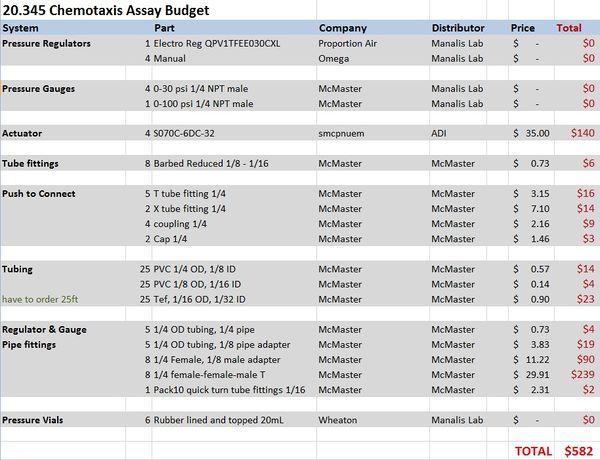
Materials and budget
Microinjector Microfluidic Device
Flow-free, or ‘stopped-flow’, chemotaxis assays allow for the analysis of individual bacterial movement in the absence of shear forces and relies on natural diffusion to generate a gradient. In this approach, fluid flow is only used to set up an initial gradient, but is stopped, allowing the gradient to evolve by diffusion alone. These devices produce unsteady gradients that are advantageous in representing environmental conditions of nutrients especially in marine bacteria and in characterizing bacterial responses to wide range of concentration and gradients within a single experiement. The bacterial distribution is measured by recording the kinetic paths of individual bacteria using videomicroscopy. An example of this approach is Seymour et al’s microinjector microfluidic device
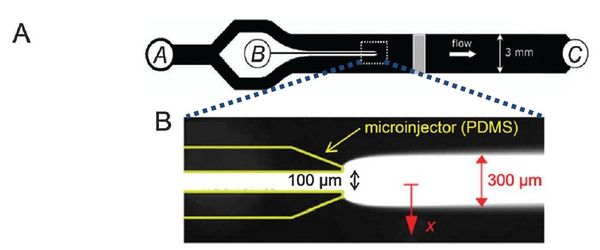
Dimmension
- Microinjector Channel
- width: 100μm
- depth: 50μm
- length:
- Main Channel
- width: 3mm
- depth: 50μm
- length:
Microscope
The imaging microscope will be a brightfield and fluorescent microscope as shown in Fig 3. A CCD camera will acquire images at a given rate and the kinetic paths will be tracked using particle tracking algorithms.
- Camera (Allied Manta G032B): 7.4μm sqr pixels, 656 x 492 pixels
- Field of View: 484μm x 366μm (10x objective w/200mm lens => 10x magnification)
- Red LED illumination
Pneumatic System Design
The pneumatic system is composed of manual and electro forward pressure regulators, solenoid actuators, pneumatic tubing, and circuitry to
Basic Design
The fundamental principle behind this system is that changes in pressures causes fluid flow and when the pressure equalize to the same 'ground' pressure, fluid flow should stop. By this approach one is able to easily control fluid flow of the entire channel and the flow rates into inlets A and B to control the width of the chemoeffector band, dependent on the ratio of P_A and P_B. Solenoid actuators, controlled by LabView, to switch from the 'flow' pressure state to the 'stopped' state.
Circuit Element Design
Experiment 1:
- By Emmanuel Quiroz