Difference between revisions of "Lab 1 Report -- Nathan S Lachenmyer"
From Course Wiki
(→E Coli) |
|||
| Line 1: | Line 1: | ||
| + | =Goals= | ||
| + | * Familiarize myself with optical trapping system | ||
| + | * Learn culture e coli, trap them! | ||
| + | |||
=Calibration= | =Calibration= | ||
All calibrations and experiments done at 20 mW. | All calibrations and experiments done at 20 mW. | ||
| Line 32: | Line 36: | ||
|} | |} | ||
| − | + | =Experiments= | |
| − | + | ==E Coli== | |
* Learned to culture E Coli (sort of) | * Learned to culture E Coli (sort of) | ||
* Examined the various cultures to determine which ones had the fastest spinners / spinners in the appropriate direction with Steve | * Examined the various cultures to determine which ones had the fastest spinners / spinners in the appropriate direction with Steve | ||
| Line 46: | Line 50: | ||
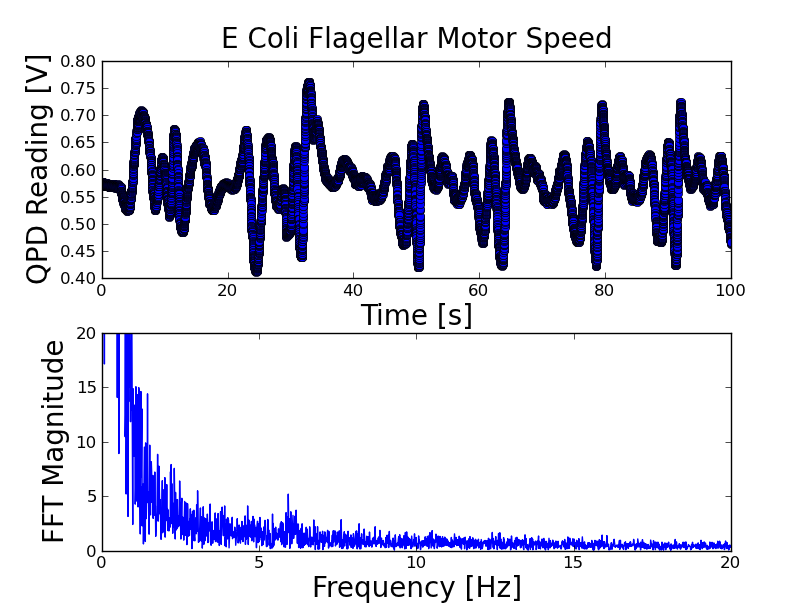
Doing an FFT analysis of the QPD spectrum, I find that the low-frequency components are pretty noisy (unfortunately). However, there is a small peak at approximately 6 Hz -- according to the appleyard paper, the e coli flagellar motor spin frequency is approximately 4-10 Hz, so this is in good agreement. | Doing an FFT analysis of the QPD spectrum, I find that the low-frequency components are pretty noisy (unfortunately). However, there is a small peak at approximately 6 Hz -- according to the appleyard paper, the e coli flagellar motor spin frequency is approximately 4-10 Hz, so this is in good agreement. | ||
| − | + | ==DNA Tethers== | |
* Learned to make the DNA tethers | * Learned to make the DNA tethers | ||
* Was able to trap a microsphere tethered to the slide via DNA | * Was able to trap a microsphere tethered to the slide via DNA | ||
| − | + | * | |
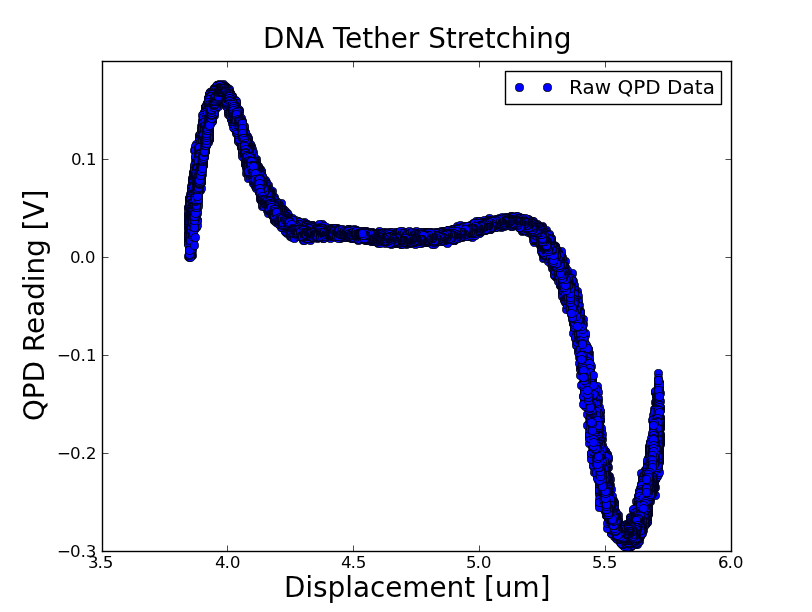
[[File:dna_raw.png]] | [[File:dna_raw.png]] | ||
Stretchiness of the DNA: | Stretchiness of the DNA: | ||
Revision as of 17:40, 12 March 2012
Contents
Goals
- Familiarize myself with optical trapping system
- Learn culture e coli, trap them!
Calibration
All calibrations and experiments done at 20 mW.
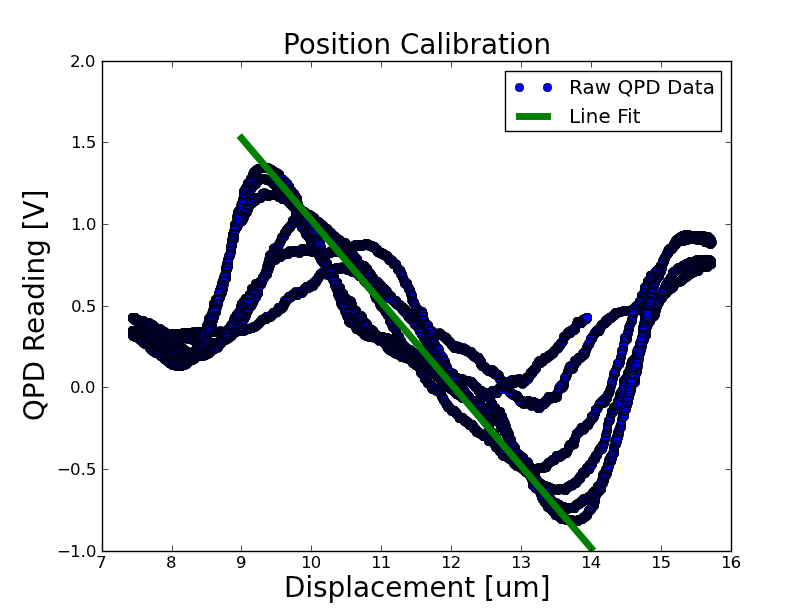
Position Calibration
- Learned to make microfluidic devices out of double-stick tape and a slide -- this was really neat!
- Made two samples with 1 um microspheres
- One sample with free-floating spheres (in H2O)
- Another one with 'stuck' spheres in NaCl (I cheated :( )
- Took a position calibration on the optical trap setup, seen below
calibration: 502463 Volts / m = 1.99 um / volt
Trap Stiffness Calibration
- Did all three versions of the trap calibration, resulting in the following trap spring constants:
| Method | Trap Stiffness (pN / nm) |
|---|---|
| Stokes | 2.26e-5 pN/nm |
| Equipartition Theorem | 1.23e-5 pN/nm |
| PSD | *Need Bandwidth of DAQ* |
Experiments
E Coli
- Learned to culture E Coli (sort of)
- Examined the various cultures to determine which ones had the fastest spinners / spinners in the appropriate direction with Steve
- E Coli weren't spinning very well -- swapped out the blue LED for a Red LED to determine if the wavelength made a difference
- As far as we could tell, the LED color made no difference
- We also couldn't figure out why the E Coli were spinning so slowly
- Worked with Steve to cut the flagella of E Coli by drawing them in and out of a pipette multiple times
- This improved the spinning frequency of the E Coli!
Doing an FFT analysis of the QPD spectrum, I find that the low-frequency components are pretty noisy (unfortunately). However, there is a small peak at approximately 6 Hz -- according to the appleyard paper, the e coli flagellar motor spin frequency is approximately 4-10 Hz, so this is in good agreement.
DNA Tethers
- Learned to make the DNA tethers
- Was able to trap a microsphere tethered to the slide via DNA
Stretchiness of the DNA: