Difference between revisions of "Assignment 2: fluorescence microscopy"
(→Electromagnetic and molecular energy levels) |
|||
| Line 64: | Line 64: | ||
===Electromagnetic and molecular energy levels=== | ===Electromagnetic and molecular energy levels=== | ||
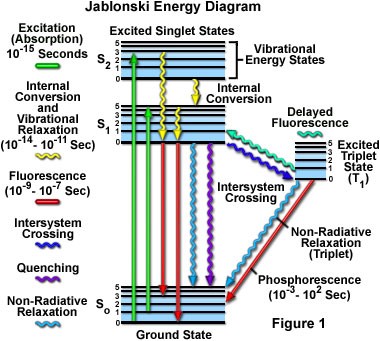
| − | + | In 1933, Aleksander Jablonski published a simple diagram that depicts the series of energy transitions that give rise to fluorescence and phosphorescence. The vertical axis represents energy. The diagram is usually divided into columns, with each column representing a different ''spin multiplicity''. | |
| − | In 1933, Aleksander Jablonski published a diagram | + | |
| + | Molecular energy levels are quantized. Allowable levels are shown by a horizontal line. Thicker horizontal lines denote the lowest energy for a given electronic state. Finer lines show vibrionic energy state. The ground state S<sub>0</sub> appears at the bottom of the diagram. | ||
| + | |||
| + | on the vertical axis and ''spin multiplicity'' on the horizontal axis. | ||
| + | <center> | ||
| + | [[File:Jablonski diagram.png|Jablonski diagram.]] | ||
| + | </center> | ||
It's not a coincidence that fluorescence takes place for the most part in visible wavelengths. Einstein | It's not a coincidence that fluorescence takes place for the most part in visible wavelengths. Einstein | ||
Revision as of 03:06, 21 August 2017
|
|
|
|---|
Overview
The phenomenon of fluorescence has beguiled both sober and inebriated witnesses since at least the late sixteenth century. Some of the earliest observers noticed an unusual blue tint in water that had been infused with the wood of a Mexican tree called Coatli. Other naturally-occurring fluorescent and phosphorescent materials such as Bologna Stone (baryte), fluorite, and quinine sparked considerable interest from some the most preeminent scientists in all of history. Galileo Galilei, Robert Boyle, David Brewster, John Herschel, and that trippy dude from your high school all spent time contemplating the curious nature and simple beauty of fluorescence.
The brilliant physicist and mathemetician George Stokes conducted a particularly clever experiment in which he used a prism to separate sunlight into its component colors. As he moved a vial of quinine sulfate from color to color, Stokes noted that quinine fluoresces only when illuminated by ultraviolet light — the invisible part of the rainbow just beyond the majestic blue band. The vial of quinine did not glow when Stokes placed the it any other part of the rainbow. Because ultraviolet illumination gave rise to blue light, Stokes concluded that fluorescent emission has a longer wavelength than the excitation. This observation turns out to be spot on, and in his honor the difference in wavelength between the excitation and emission peak a fluorophore's spectrum is called the Stokes shift.
Reconciling Stokes' observation with the prevailing theory of light at the time of his experiment proved to be extraordinarily difficult. In classical electromagnetic theory, the power of a wave depends on its intensity, not its frequency. Bright light has a larger electric field than dim light, so in classical theory bright light should excite an electron more vigorously than dim light. But that prediction is at odds with the results of Stokes' experiment. Quinine is excited by UV light and none of the other colors, no matter how bright the light.[1] In order to explain this, Albert Einstein had to dream up a whole new branch of physics: the quantum theory. Einstein introduced the basic that light comes in discrete packets in one of his "miracle year" papers in 1905 with the dense title of Concerning an Heuristic Point of View Toward the Emission and Transformation of light. This is a lovely paper, and you should put it on your reading list. In it, Einstein posited that light is made of discrete packets of energy (which we now call photons). The energy of photon depends only on its color and is given by the equation:
- $ E=h\nu=\frac{hc}{\lambda} $,
where ν is wavelength and h is the Planck constant approximately equal to 6.626070040(81)×10−34 J⋅s. In Section 7 of the paper, Einstein offered a persuasive theoretical argument for the correctness of Stokes' rule: "If the fluorescent substance is not a perpetual source of energy, the principle of conservation of energy requires that the energy of an emitted energy quantum cannot be greater than of the incident light quantum.."[2]
Electromagnetic and molecular energy levels
In 1933, Aleksander Jablonski published a simple diagram that depicts the series of energy transitions that give rise to fluorescence and phosphorescence. The vertical axis represents energy. The diagram is usually divided into columns, with each column representing a different spin multiplicity.
Molecular energy levels are quantized. Allowable levels are shown by a horizontal line. Thicker horizontal lines denote the lowest energy for a given electronic state. Finer lines show vibrionic energy state. The ground state S0 appears at the bottom of the diagram.
on the vertical axis and spin multiplicity on the horizontal axis.
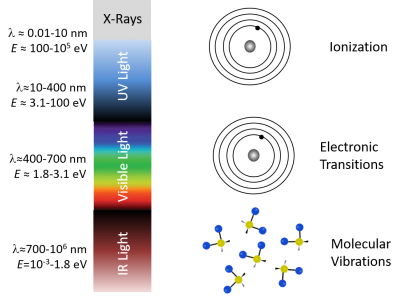
It's not a coincidence that fluorescence takes place for the most part in visible wavelengths. Einstein When it comes to As summarized in the diagram below, single photons with wavelengths longer than about 700 nm have energies less than about 1.8 electron volts (2.88×10-19 J), which roughly corresponds to the energy range of vibrational modes of molecules. Visible photons (λ= 400 - 700 nm) have energies in the same range as the differences between in energy levels of electrons bound to a molecule. X-rays (0.01 - 10 nm) have more than enough energy to completely remove an electron. Ultraviolet light bridges the gap.
20.309_130828_FluoAbsorbEmitSpectra2.png
Some naturally fluorescent molecules like fluorescein and quinine have important biological applications on their own. But a key breakthrough in the early 1940s dramatically increased the utility and importance of fluorescence in biology. The key idea was to attach a fluorescent molecule to an antibody. The combination of the two offers spectacular contrast and specificity in microscopic studies of biological samples. Today, fluorescent immunostaining is one of the most important and frequently used techniques in all of biological research.
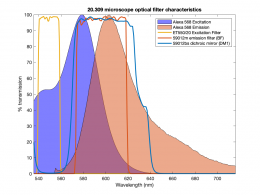
In this part of the lab, you will add the hardware necessary to make epi-illuminated fluorescent images with your microscope and make a few test images of plastic, fluorescent beads. A typical spectrum is shown on the right. The excitation light source is a 5 mW diode laser with a nominal wavelength of 532 nm — a striking, brilliant green. Emission is in the red/orange range. To make the correction for nonuniform illumination, you will also make images of a uniform fluorescence reference slide and a dark image with the illuminator turned off.
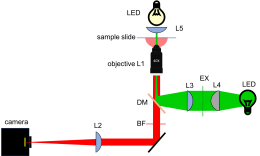
As shown in the block diagram, the major components required for fluorescence imaging are an illuminator (laser, L3, L4, and L5), dichroic mirror (DM), and emission filter (BF). The illuminator provides light in the appropriate wavelength range to excite the fluorescent molecules in the sample. Fluorescence microscopes that use broadand light sources such as arc lamps require an additional filter called an excitation filter to limit the wavelengths in the illumination to the proper range. Because lasers emit light in a very narrow range of wavelengths, an excitation filter is unnecessary.
Excitation light comes from beneath the sample, through the objective lens. A dichroic mirror directs the excitation toward the objective and sample. The mirror must reflect wavelengths in the excitation range and pass the longer wavelengths of the emitted fluorescence. In fluorescence imaging, illumination intensity is typically 5 or 6 orders of magnitude greater than emitted fluorescence, so it is crucial to filter out excitation photons as completely as possible. The dichroic mirror passes a substantial amount of green light, on the order of five percent. The barrier filter does a much better job of removing the green light, attenuating the excitation wavelengths by about 5 orders of magnitude. The barrier filter is essential for making crisp, high-contrast images.
To provide collimated illumination in the sample plane, light from the illuminator is focused at the back focal point of the objective.
Assignment details
This assignment has 2 parts:
Cite error: <ref> tags exist, but no <references/> tag was found