Difference between revisions of "20.109(S24):M1D2"
Noreen Lyell (Talk | contribs) (→Navigation links) |
Noreen Lyell (Talk | contribs) (→Navigation links) |
||
| Line 133: | Line 133: | ||
==Navigation links== | ==Navigation links== | ||
Next day: [[20.109(S24):M1D3 |Assess purity and concentration of expressed protein]] <br> | Next day: [[20.109(S24):M1D3 |Assess purity and concentration of expressed protein]] <br> | ||
| − | Previous day: [[20.109(S24):M1D1 | Complete in-silico cloning of protein expression | + | Previous day: [[20.109(S24):M1D1 | Complete in-silico cloning of protein expression vector]]<br> |
Latest revision as of 16:26, 5 February 2024
Introduction
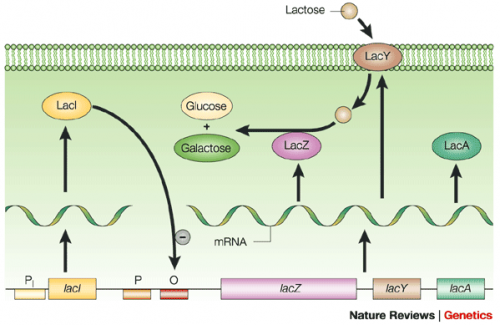
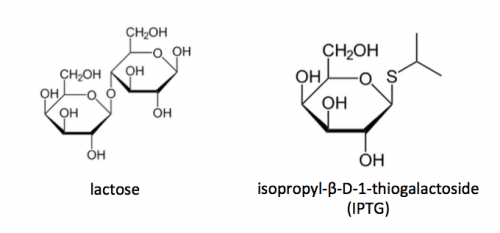
To induce production of MAX protein from the expression plasmid that was 'cloned' in the previous laboratory session, a lactose-analogue isopropyl β-D-1-thiogalactopyranoside (IPTG) was used to induce expression in BL21(DE3) E. coli bacterial cells. The use of IPTG to induce protein expression is based on the native lac operon used for lactose metabolism in bacterial cells.
The lac operon is composed of four genes: lacI, lacZ, lacY, and lacA. When lactose is absent, LacI (the protein encoded by lacI) binds to the operator sequence (O) upstream of lacZYA. In the presence of lactose, LacI and lactose form a complex which relieves repression of lacZYA transcription. LacZ is a β-galactosidase that cleaves lactose resulting in glucose and galactose. LacY, a β-galactoside permease, facilitates the transport of lactose across the cell membrane, and LacA, a β-galactoside transacetylase, transfers an acetyl group from acetyl-CoA to β-galactosides.The native lac operon is a powerful tool in engineering protein expression systems because it enables researchers to control gene expression using inducer molecules. The lacZYA genes are only expressed when lactose is present. If a gene of interest is cloned downstream of the operator sequence, the expression of this gene can be controlled by LacI repression and lactose derepression. To further control the system for protein expression, IPTG is used as a lactose-analog as it is not metabolized by the cells.
Today you will isolate the expressed MAX protein from the bacterial cells. Remember that the MAX gene sequence was synthesized using a gBlock and 6xHis-tag was added to the DNA sequence. The resultant protein is therefore His-tagged. Histidine has several interesting properties, notably its near-neutral pKa, and His-rich peptides are promiscuous binders, particularly to metals. (For example, histidine side chains help coordinate iron molecules in hemoglobin.)
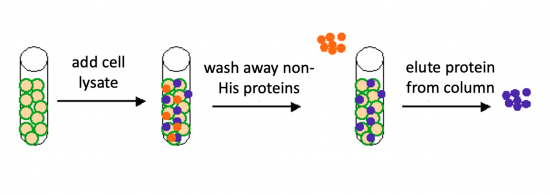
To purify the MAX proteins present in the bacterial cell, you will use a nickel-agarose resin. The 6xHis-tag on the MAX protein will bind to the nickel-coated resin, while the other cellular protein will pass through the resin. Remember, the BL21(DE3) cells are not only producing the MAX protein, but also the proteins needed for cellular function and survival. Imidazole is a compound that is also able to bind to nickel and washing the resin with a low concentration solution promotes competition for binding between the imidazole and bound proteins for the nickel-coated resin. Proteins that are non-specifically bound will have a lower affinity for the nickel than imidazole and be washed from the column, whereas the 6xHis-tagged MAX will remain adhered to the nickel-agarose resin. To elute the MAX protein from the nickel-coated resin, a high concentration of imidazole is used to out-compete the 6xHis-tag for binding.
Protocols
Part 1: Induce expression of MAX-6xHis
For timing reasons, the induction steps were completed prior to class. So you understand how the cell pellets you will use for the protein purification, the steps are shown in the video and protocol below.
To ensure the steps included below are clear, please watch the video tutorial linked here: [Bacterial Induction]. The steps are detailed below so you can follow along!
- Inoculated 5 mL of LB media containing 50 μg/mL kanamycin with a colony of BL21(DE3) cells transformed with pET-28a_MAX-6xHis.
- Incubated the culture overnight at 37 °C with shaking at 220 rpm.
- Dilute the overnight culture 1:100 in 50 mL of fresh LB media containing 50 μg/mL kanamycin.
- Incubate at 37 °C until the OD600 = 0.6 with shaking at 220 rpm.
- To induce MAX-6xHis protein expression, add IPTG to a final concentration of 0.1 μM.
- Incubate 2.5 hrs at at 37 °C with shaking at 100 rpm.
- To harvest the cells, centrifuge the culture at 4000 g for 15 min at 4 °C.
- Cell pellets were stored at -80 °C until used for purification.
In your laboratory notebook, complete the following:
- Calculate the volume of kanamycin stock that was added to the LB broth in Step #1. In Step #3.
- Concentration of kanamycin stock = 50 mg/mL.
- Calculate the volume of IPTG stock that was added to the LB broth in Step #5.
- Concentration of IPTG stock = 1 mM.
Part 2: Purify MAX-6xHis protein
To ensure the steps required for purifying the protein are clear, the Teaching Assistant will provide a live demonstration of this process.
Lyse BL21(DE3) cells expressing pET-28a_MAX-6xHis
- Retrieve a conical tube containing a BL21(DE3) pET-28a_MAX-6xHis cell pellet from the -80 °C freezer and leave it on your bench to thaw.
- To lyse the cells, resuspend the pellet in B-Per bacterial extraction reagent at 2 mL / cell pellet.
- To the cell suspension add:
- lysozyme at 2 μL / mL of B-Per bacterial extraction reagent
- DNAse at 2 μL / mL of B-Per bacterial extraction reagent
- protease inhibitor at 2 μL / mL of B-Per bacterial extraction reagent
- Solubilize the cell pellet in lysis buffer and vortex to mix.
- Incubate cell pellet in lysis buffer at room temperature for 15 min on nutator.
- Transfer lysate into a fresh 15 mL conical tube.
- This will allow us to fit all of the class samples into the centrifuge.
- To pellet the cell debris, centrifuge the lysate at 4,000 rcf for 45 min at 4 °C.
- Complete Part 3: Electrophorese confirmation digests during the centrifugation.
Prepare Ni-NTA affinity column
- Obtain a 500 μL aliquot of 50% slurry (Ni-NTA resin) and mix the slurry by inverting the tube several times.
- The slurry is the Ni-NTA column matrix.
- Centrifuge the slurry for 30 sec then remove the supernatent.
- To wash the slurry, add 500 μL of 1X PBS and invert the tube 3 times.
- Add the slurry to the column and allow the 1X PBS to run through the column.
- Be sure a beaker is placed under the column to collect the waste!
- When the PBS has flowed through, cap the bottom and the top of the column until you are ready to add the cell lysate.
Purify MAX-6xHis from cell lysate
- Aliquot 30 μL of the supernatent from the tube containing the centrifuged lysate to a fresh microcentrifuge tube.
- Label the microcentrifuge tube containing the aliquot as "lysate" and give it to the Instructor! This aliquot will be used later when protein expression and purity are examined.
- Label the conical tube containing the cell pellet as "pellet" and give it to the Instructor! This pellet will be used later when protein expression and purity are examined.
- Pipet the remaining supernatent into the prepared Ni-NTA affinity column.
- Be sure that the bottom of the column is capped!
- Pipette the resin / cell supernatent to mix and incubate at room temperature on a nutator for 15 min.
- Hold a microcentrifuge tube under the column, then remove the bottom cap from the column and collect the liquid that leaves the column.
- Label the microcentrifuge tube as "flowthrough" and give it to the Instructor! This aliquot will be used later when protein expression and purity are examined.
- To wash the Ni-NTA affinity column, add 10 mL of wash buffer.
- Hold a microcentrifuge tube under the column, then remove the bottom cap from the column and collect ~250 μL of the liquid that leaves the column.
- Label the microcentrifuge tube as "wash" and give it to the Instructor! This aliquot will be used later when protein expression and purity are examined.
- To elute the MAX-6xHis protein from the affinity column, add 1 mL of elution buffer and incubate at room temperature for 5 min.
- Hold a microcentrifuge tube under the column, then remove the bottom cap from the column and collect the entire 1 mL of the liquid that leaves the column.
- Label the microcentrifuge tube as "elution" and give it to the Instructor! This aliquot will be used later when protein expression and purity are examined.
- Lastly, resuspend the slurry from the Ni-NTA affinity column in 250 μL 1X PBS and transfer to a fresh microcentrifuge tube.
- Label the microcentrifuge tube as "slurry" and give it to the Instructor! This aliquot will be used later when protein expression and purity are examined.
In your laboratory notebook, complete the following:
- At several steps in the protein purification procedure, samples are collected that will be used later when protein expression and purity are examined. Consider why each of the samples listed below are saved as controls to measure the success of the purification.
- The lysate from Step #1.
- The pellet from Step #1.
- The flowthrough from Step #4.
- The wash from Step #5.
- The slurry from Step #7.
- What is occurring during the incubation in Step #3?
Part 3: Electrophorese confirmation digests
Electrophoresis is a technique that separates large molecules by size using an applied electrical field and a sieving matrix. DNA, RNA and proteins are the molecules most often studied with this technique; agarose and acrylamide gels are the two most common sieves. The molecules to be separated enter the matrix through a well at one end and are pulled through the matrix when a current is applied across it. The larger molecules get entwined in the matrix and are stalled; the smaller molecules wind through the matrix more easily and travel farther away from the well. The distance a DNA fragment travels is inversely proportional to the log of its length. Over time fragments of similar length accumulate into “bands” in the gel. Higher concentrations of agarose can be used to resolve smaller DNA fragments.
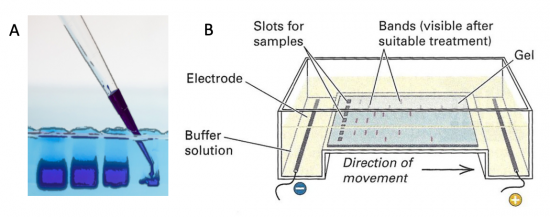
DNA and RNA are negatively charged molecules due to their phosphate backbone, and they naturally travel toward the positive electrode at the far end of the gel. Today you will separate DNA fragments using an agarose matrix. Agarose is a polymer that comes from seaweed. To prepare these gels, agarose and 1X TAE buffer (Tris base, acetic acid, and EDTA) are microwaved until the agarose is melted and fully dissolved. The molten agar is then poured into a horizontal casting tray, and a comb is added. Once the agar has solidified, the comb is removed, leaving wells into which the DNA samples can be loaded.
For the digests that were prepared in the previous laboratory session, a 1% agarose gel with SYBR Safe DNA stain was used to separate the DNA fragments in the four digest reactions. In addition, a well was loaded with a molecular weight marker (also called a DNA ladder) to determine the size of the fragments.
- Add 5 μL of 6x loading dye to the digests.
- Loading dye contains bromophenol blue as a tracking dye, which enables you to follow the progress of the electrophoresis.
- Glycerol is also included to weight the samples such that the liquid sinks into well.
- Flick the eppendorf tubes to mix the contents, then quick spin them in the microfuge to bring the contents of the tubes to the bottom.
- Load 25 μL of each digest into the gel, as well as 5 μL of 1kb DNA ladder.
- Be sure to record the order in which you load your samples!
- To load your samples, draw the volume listed above into the tip of your P200 or P20. Lower the tip below the surface of the buffer and directly over the well. Avoid lowering the tip too far into the well itself so as to not puncture the well. Expel your sample slowly into the well. Do not release the pipet plunger until after you have removed the tip from the gel box (or you'll draw your sample back into the tip!).
- Once all the samples have been loaded, attach the gel box to the power supply and electrophorese the gel at 125 V for 45 minutes.
- Lastly, visualize the DNA fragments in the agarose gel using the gel documentation system.
Reagents list
- Luria-Bertani broth (LB) (from Difco)
- kanamycin (from Sigma)
- isopropyl β-d-1-thiogalactopyranoside (IPTG) (from Sigma)
- 2x B-Per bacterial protein extraction reagent (from ThermoFisher)
- lysozyme (from Sigma)
- DNAse (from Sigma)
- protease inhibitor (from Sigma)
- phosphate saline buffer (PBS) (from VWR)
- Ni-NTA agarose (from Qiagen)
- Wash buffer: 100 mM HEPES (pH = 7.4), 500 mM NaCl, 50 mM imidazole
- Elution buffer: 100 mM HEPES (pH = 7.4), 500 mM NaCl, 250 mM imidazole
- imidazole (from Sigma)
Next day: Assess purity and concentration of expressed protein