Difference between revisions of "20.109(S22):M1D5"
Noreen Lyell (Talk | contribs) (→Protocols) |
Noreen Lyell (Talk | contribs) (→Protocols) |
||
| Line 12: | Line 12: | ||
Our communication instructor, Dr. Prerna Bhargava, will join us today for a discussion on writing figure titles and captions. | Our communication instructor, Dr. Prerna Bhargava, will join us today for a discussion on writing figure titles and captions. | ||
| − | ===Part 2: | + | ===Part 2: Concentrate TDP43-RRM12 protein elution=== |
| − | ===Part 3: Draft Data summary slide for protein purity and concentration results=== | + | Before evaluating the purity and concentration of the TDP43-RRM12 protein, it is important to concentrate the protein solution (the protein solution is the elution from the previous laboratory session!). Concentrating the protein eliminates excess buffer and contaminants. To do this, a centrifugal filter with a 3 kDa cutoff will be used. The cutoff value refers to the size of the molecules that are able to pass through the filter -- molecules smaller than 3 kDa flowthrough the filter whereas molecules larger than 3 kDa are retained in the reservoir above the filter. One critical consideration when selecting a centrifugal filter is the molecular weight of the protein of interest. The protein should be larger than the cutoff value to ensure it is retained in the reservoir above the filter. |
| + | |||
| + | #Retrieve the desalted "elution" sample you collected in the previous laboratory session. | ||
| + | #Aliquot 30 μL of the elution to a fresh microcentrifuge tube. | ||
| + | #*'''Label the microcentrifuge tube containing the aliquot as "elution" and give it to the Instructor! This aliquot will be used later when protein expression and purity are examined.''' | ||
| + | #Add the remaining contents of the original elution tube to the centrifugal filter unit. | ||
| + | #*Remove the cap from the filter unit, then pipette the TDP43-RRM12 protein solution into the filter unit and replace the cap. | ||
| + | #Centrifuge at 4500 g for 20 minutes. | ||
| + | #After centrifugation, transfer the liquid from the top of the filter unit to a fresh microcentrifuge tube. | ||
| + | #*'''Label the microcentrifuge tube containing the liquid as "concentrated protein" and give it to the Instructor! This aliquot will be used later when protein expression and purity are examined.''' | ||
| + | #*This tube contains the purified and concentrated TDP43-RRM12 protein that will be used for the BCA assay (Part 3) and the SMM experiment (next laboratory session). | ||
| + | #From the "concentrated protein" tube, aliquot 30 μL to a fresh microcentrifuge tube. | ||
| + | #*'''Label the microcentrifuge tube containing the concentrated protein as "concentrated protein for gel" and give it to the Instructor! This aliquot will be used later when protein expression and purity are examined.''' | ||
| + | #*This tube contains an aliquot of the purified and concentrated TDP43-RRM12 protein that will be used for SDS-PAGE analysis. | ||
| + | |||
| + | |||
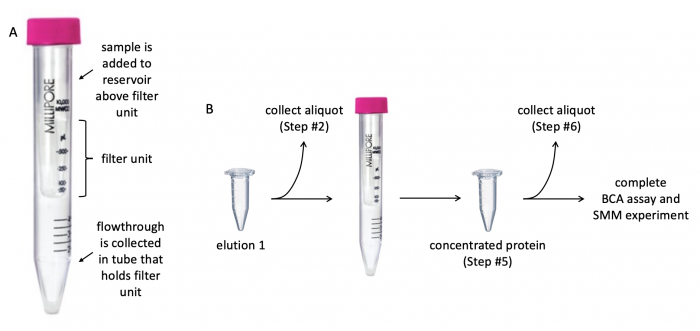
| + | [[File:Fa20 M2D3 protein concentration.png|center|700px|thumb|'''Diagram of centrifugal filter and concentration protocol.''' (A) Centrifugal filter with 3 kDa cutoff is used to concentrate protein solution and remove contaminants. (B) Aliquots are collected at different steps of concentration procedure to assess protein expression and purity.]] | ||
| + | |||
| + | ===Part 3: Perform aggregation assay=== | ||
| + | |||
| + | ===Part 4: Draft Data summary slide for protein purity and concentration results=== | ||
To get a headstart and further feedback from the Instructors, you will draft the slide that will present the TDP43 protein purification data for your Data summary today in class. With your partner, use the template below, the Instructor comments from your previous homework assignments, and the helpful hints from the Comm Lab workshop to craft a data slide with the required elements. | To get a headstart and further feedback from the Instructors, you will draft the slide that will present the TDP43 protein purification data for your Data summary today in class. With your partner, use the template below, the Instructor comments from your previous homework assignments, and the helpful hints from the Comm Lab workshop to craft a data slide with the required elements. | ||
Revision as of 16:40, 14 February 2022
Contents
Introduction
Protocols
Part 1: Participate in Communication Lab workshop
Our communication instructor, Dr. Prerna Bhargava, will join us today for a discussion on writing figure titles and captions.
Part 2: Concentrate TDP43-RRM12 protein elution
Before evaluating the purity and concentration of the TDP43-RRM12 protein, it is important to concentrate the protein solution (the protein solution is the elution from the previous laboratory session!). Concentrating the protein eliminates excess buffer and contaminants. To do this, a centrifugal filter with a 3 kDa cutoff will be used. The cutoff value refers to the size of the molecules that are able to pass through the filter -- molecules smaller than 3 kDa flowthrough the filter whereas molecules larger than 3 kDa are retained in the reservoir above the filter. One critical consideration when selecting a centrifugal filter is the molecular weight of the protein of interest. The protein should be larger than the cutoff value to ensure it is retained in the reservoir above the filter.
- Retrieve the desalted "elution" sample you collected in the previous laboratory session.
- Aliquot 30 μL of the elution to a fresh microcentrifuge tube.
- Label the microcentrifuge tube containing the aliquot as "elution" and give it to the Instructor! This aliquot will be used later when protein expression and purity are examined.
- Add the remaining contents of the original elution tube to the centrifugal filter unit.
- Remove the cap from the filter unit, then pipette the TDP43-RRM12 protein solution into the filter unit and replace the cap.
- Centrifuge at 4500 g for 20 minutes.
- After centrifugation, transfer the liquid from the top of the filter unit to a fresh microcentrifuge tube.
- Label the microcentrifuge tube containing the liquid as "concentrated protein" and give it to the Instructor! This aliquot will be used later when protein expression and purity are examined.
- This tube contains the purified and concentrated TDP43-RRM12 protein that will be used for the BCA assay (Part 3) and the SMM experiment (next laboratory session).
- From the "concentrated protein" tube, aliquot 30 μL to a fresh microcentrifuge tube.
- Label the microcentrifuge tube containing the concentrated protein as "concentrated protein for gel" and give it to the Instructor! This aliquot will be used later when protein expression and purity are examined.
- This tube contains an aliquot of the purified and concentrated TDP43-RRM12 protein that will be used for SDS-PAGE analysis.
Part 3: Perform aggregation assay
Part 4: Draft Data summary slide for protein purity and concentration results
To get a headstart and further feedback from the Instructors, you will draft the slide that will present the TDP43 protein purification data for your Data summary today in class. With your partner, use the template below, the Instructor comments from your previous homework assignments, and the helpful hints from the Comm Lab workshop to craft a data slide with the required elements.
Individually you and your laboratory partner each crafted figures (with a title and caption) using the TDP43 protein purification results. In this exercise you will come together to decide how to best present these data for the Data summary!
Reagents list
Next day: Learn best practices for mammalian cell culture and seed CAD cells for TDP43-localization experiment