20.109(S10):Start-up biomaterials engineering (Day1)
Contents
Introduction
Today we will continue the discussion that we began in lecture about cell-biomaterial interactions and cartilage tissue engineering, with the ultimate goal of designing an experiment probing chondrocyte phenotype development and/or maintenance. Several papers on chondrocyte tissue culture and cartilage tissue engineering will be available in class, and you are also welcome to search the scientific literature on your own for further ideas. You will also get some practice with cell culture today, to prepare you for beginning your experiment next time.
Protocols
Half the class today will start in the cell culture facility and half will start with experimental design. Midway through class, you'll switch places.
Part 1: Practice cell culture
Background
In the past century, we have learned a tremendous amount by studying the behavior of mammalian cells maintained in the laboratory. Tissue culture was originally developed about 100 years ago as a method for learning about mammalian biology. The term tissue culture was originally coined because people were doing exactly that, extracting tissue and letting it live in a dish for a short time. Today, most tissue culture experiments are done using cells rather than tissues. Much of what we know about cancer, heritable diseases, and the effects of the environment on human health has been derived from studies of cultured cells.
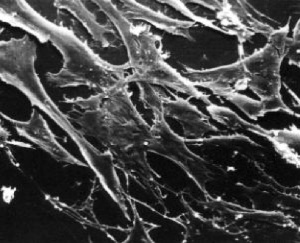
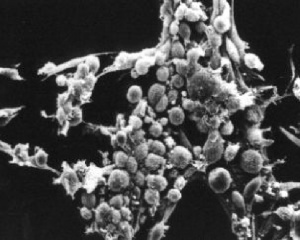
What types of cells do people study, and where do they come from? Cells that come from a tissue are called primary cells, because they come directly from an animal. It is very difficult to culture primary cells, largely because primary cells that are placed in culture divide only a limited number of times. This limitation in the lifespan of cultured primary cells, called the Hayflick limit, is a problem because it requires a researcher to constantly remove tissues from animals in order to complete a study. Cell isolation processes can be quite labour-intensive, and also can complicate data analysis due to inherent animal-to-animal variation. To get around this problem, people have studied cells that are immortal, which means that they can divide indefinitely. Some inherent cell-to-cell variation still exists in such cells. Moreover, the genetic changes that cause immortality may affect experimental outcomes.
One type of familiar immortalized cell is the cancer cell. Tumor cells continuously divide allowing cancer to invade tissues and proliferate. Cancer cells behave the same way in culture, and under the right conditions, cells can be taken from a tumor and divide indefinitely in culture. Another type of immortalized cell is the embryonic stem cell. Embryonic stem cells are derived from an early stage embryo, and these cells are completely undifferentiated and pluripotent, which means that under the right conditions, they can become any mammalian cell type. Mouse embryonic stem cells have become a valuable research tool, and it is this cell type that we will be using for our practice cell culture today.
The art of tissue culture lies in the ability to create conditions that are similar to what a cell would experience in an animal, namely 37°C and neutral pH. Blood nourishes the cells in an animal, and blood components are used to feed cells in culture. Serum, the cell-free (and clotting-factor free) component of blood, contains many of the factors necessary to support the growth of cells outside the animal. Consequently, serum is frequently added to tissue culture medium, although serum-free media exist and support some types of cultured cells.
Cultured mammalian cells must grow in a germ-free environment and researchers using tissue culture must be skilled in sterile technique. Germs double very quickly relative to mammalian cells. An average mammalian cells doubles about once per day whereas many common bacteria can double every 20 minutes under optimal conditions. Consequently, if you put 100 mammalian cells and 1 bacteria together in a dish, within 24 hours you would have ~200 unhappy mammalian cells, and about 100 million happy bacteria! Needless to say, you would not find it very useful to continue to study the behavior of your mammalian cells under these conditions!
One major objective for this experimental module is for you to learn how to perform tissue culture. Today you will learn how to get mouse embryonic stem cells to grow in a dish and also how to prevent contaminants from getting into your cell cultures. Next time you will set up 3D cultures of bovine-derived cells in alginate beads.
Part 2: Experiment design
The overall goal of this module is to test the effect of the surrounding environment on cell phenotype. In particular, you will work with primary chondrocytes and/or mesenchymal stem cells in 3D gel culture. The specific aspects of phenotype assayed will be collagen I and collagen II transcript and protein levels (these are markers for cell type), as well as the general cell characteristic of viability. You are free to propose one other assay if you wish, as you will likely have some extra time in the latter part of the module. One possibility is a DMMB colorimetric assay for proteoglycan content. You will be able to compare some of the data in your 3D culture experiments with control data from freshly isolated chondrocytes and stem cells.
Each pair of you will test two samples. Both samples will be grown in 3D alginate bead culture, and should have one parameter varied between them. For example, you might try changing the mechanical properties of the beads (how would you do this?), or the cell density within the beads. Be as creative as you like! If your protocol requires a new reagent or equipment to be ordered, we will do our best to get it in time. Of course, two samples is not very many for determining a trend. You are more than welcome to join up with another group or two in order to expand the range of the parameter you are testing (e.g., testing four cell densities instead of two). If everyone wants to test something different, that’s okay too.
Most of you should explore conditions for maintaining or destroying chondrocyte phenotype - recall from lecture that chondrocytes grown without proper signals, for example in simple monolayer culture, tend to de-differentiate to a fibroblastic phenotype over time. We will also have some mesenchymal stem cells for a few groups to work with, and investigate conditions that promote chondrogenesis. Please see the teaching faculty with your proposed experiment, as we have a limited amount of each cell type.
Per 3D sample, you will prepare 1 mL of alginate beads (thus a cell density of 5 million cells per mL would require 5 million cells per sample, 10 million total).
Materials available for 3D culture
| Alginate company | Alginate name | Viscosity | G/M Ratio |
| Sigma Aldrich | "low viscosity" | 250 cps at 2% | "high M" |
| FMC Biopolymer | Protanal LF 120M | 70-150 cps at 1% | ~40/60 |
| FMC Biopolymer | Protanal LF 10/60 | 20-70 cps at 1% | ~70/30 |
FMC Biopolymer alginates are samples generously donated by the company.
Collagen I and II gels are also available upon request. Keep in mind that using collagen directly will confound your protein assay results (unless you devise some controls), but not the transcript-level assay results.
Standard stem cell medium
- Expansion Medium
- Low-glucose DMEM
- 10% FCS
- Penicillin/Streptomycin
- Amphotericin B
- HEPES buffer, 10 mM (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid)
- up to 5 ng/mL bFGF (basic fibroblast growth factor)
- Differentiation Medium
- Hi-glucose DMEM
- FCS or ITS (insulin/transferrin/selenium)
- Penicillin/Streptomycin
- Amphotericin B
- Non-essential amino acids
- Sodium pyruvate
- Proline (400 μM)
- HEPES (10 mM)
- Chondrogenic factors
- TGF-beta1 (10 ng/mL)
- Dexamethasone (100 nM)
- Ascorbate
Standard chondrocyte medium
- Growth medium
- Hi-glucose DMEM
- 10% FCS (or less, with ITS)
- Penicillin/Streptomycin
- Amphotericin B
- Non-essential amino acids
- Sodium pyruvate
- Proline (400 μM)
- HEPES (10 mM)
- Ascorbate(20 μg/mL)
Part 2: Reading period (optional)
If you wish, you can use any remaining time today to familiarize yourself with and begin the major assignments for Module 3.
For next time
- Familiarize yourself with the cell culture portion of Day 2 of this module. The better prepared we all are, the less likely it is that the day will run long. The hoods will be set up for you when you come in.
- Write a two or three sentence description of your design plan and expected assay results. (Assay result expectations should be stated in a relative fashion: e.g., "we think 3D sample 1 will maintain a chondrocyte-like phenotype better than 3D sample 2, because..." You might also comment on cell viability, if you expect it to vary among your samples.)