Difference between revisions of "20.109(S09):Bacterial amplification of DNA (Day3)"
(→Introduction) |
(→Protocols) |
||
| Line 23: | Line 23: | ||
==Protocols== | ==Protocols== | ||
| + | |||
| + | ===Part 1: Agarose gel electrophoresis=== | ||
| + | |||
| + | Using a 1% agarose gel prepared by the teaching faculty, you will run four samples as well as a reference lane of molecular weight markers (also called a DNA ladder). | ||
| + | |||
| + | #Set aside 10 μL of each of your DpnI-digested products – Y64D and X#Y – in eppendorf tubes. '''Save the rest of the digested DNA, keeping it on ice.''' | ||
| + | #Retrieve the two aliquots of undigested sample that you prepared last time, and add 2 μL of loading dye to all four samples. | ||
| + | #*Loading dye contains xylene cyanol as a tracking dye to follow the progress of the electrophoresis (so you don’t run the smallest fragments off the end of your gel!) as well as glycerol to help the samples sink into the well. | ||
| + | #Flick the eppendorf tubes to mix the contents, then quick spin them in the microfuge to bring the contents of the tubes to the bottom. | ||
| + | #Load the gel in the order shown in the table below. | ||
| + | #*To load your samples, draw 10 μL into the tip of your P20. Lower the tip below the surface of the buffer and directly over the well. You risk puncturing the bottom of the well if you lower the tip too far into the well itself (puncturing well = bad!). Expel your sample into the well. Do not release the pipet plunger until after you have removed the tip from the gel box (or you'll draw your sample back into the tip!). | ||
| + | #Once all the samples have been loaded, the teaching faculty will attach the gel box to the power supply and run the gel at 125 V for 45 minutes. | ||
| + | #While the gels run, you will here from the Writing Across the Curriculum teaching faculty. If you are done early, you can skip ahead to Part 4 of the protocol. | ||
| + | |||
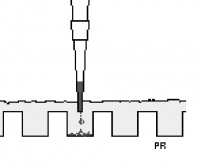
| + | [[Image:Be109gelloading.jpg|thumb|center|200px|'''Loading a gel''']] | ||
| + | |||
| + | ===Part 2: Gel analysis==== | ||
| + | |||
| + | #You will be shown how to photograph and save a digital image of your gel. | ||
| + | #In the following analysis, you will need the information for the 1 Kbp ladder you used, which is available at [http://www.neb.com/nebecomm/products/productN3232.asp this link]. | ||
| + | #First, see if you got a band at the expected size of inverse pericam in pRSET, or ~ 4 Kb | ||
| + | #If you did not get a band, consider preparing an extra transformation for that sample with 2-5x the usual recommended DNA amount. | ||
| + | #If you did get a band, estimate the approximate amount of DNA in that lane, then the concentration (based on the sample volume that you loaded). Keep this information in your notebook. | ||
| + | |||
| + | ===Part 3: Bacterial transformation=== | ||
| + | |||
| + | Each mutagenesis reaction (Y64D, and your chosen X#Y) will be transformed into competent cells called XL1-Blue and plated on separate Petri dishes. Tomorrow, two candidate colonies will be chosen from each plate. The efficiency of this mutagenesis protocol is reported to be 80 %. We will test two candidates per mutation to cover our bases, so to speak. | ||
| + | |||
| + | #Get an aliquot of competent cells from one of the teaching faculty. Keep these cells on ice at all times, allowing them to thaw slowly (over a few minutes). | ||
| + | #Label three 14 mL polypropylene round-bottom tubes as follows: (-) control Y64D, X#Y. | ||
| + | #Add 50 μL of competent cells to each tube, followed by 1 & m u ; L o f the appropriate D N A. Gently swirl (do not vortex) to mix, then incubate on ice for 10 minutes. | ||
| + | # Bring the tubes over to the 42 °C water bath, and immerse them for exactly 45 seconds according to your digital timer. | ||
| + | #Immediately return the cells to ice for 2 minutes, and take an aliquot of pre-warmed LB medium. | ||
| + | #Add 0.5 mL of warm LB to each sample, then move them to the 37 °C incubator. Ask the teaching faculty to show you how to operate the roller and balance your tubes. | ||
| + | #Allow the cells to recover and begin expressing ampicillin for 30 minutes. At the same time, pre-warm and dry three (or more, if needed) LB+AMP plates by placing them in the 37°C incubator, media side up with the lids ajar. | ||
| + | #Plate 250 μl of each transformation mix on LB+AMP plates. After dipping the glass spreader in the ethanol jar, you should pass it through the flame of the alcohol burner '''just long enough to ignite the ethanol'''. '''After letting the ethanol burn off, the spreader may still be very hot''', and it is advisable to tap it gently on a portion of the agar plate without cells in order to equilibrate it with the agar (if it sizzles, it's way too hot). Once the plates are done, wrap them with colored tape and incubate them in the 37°C incubator overnight. One of the teaching faculty will remove them from the incubator and set up liquid cultures for you to use next time. | ||
| + | |||
| + | ===Part 4: Prepare tubes for liquid O/N cultures=== | ||
| + | |||
| + | You will make your teaching faculty very happy if you contribute to their preparatory work. Please label 4 large glass test tubes with your team color and sample name. Mix 12 ml LB with 12 μL of ampicillin. Aliquot 2.5 mL of LB+Amp per tube. These will be used to set up overnight cultures for you for next time. | ||
==For next time== | ==For next time== | ||
==Reagent list== | ==Reagent list== | ||
Revision as of 15:07, 6 October 2008
Contents
Introduction
Assuming all went well, your reaction tubes from last time contain mutagenized DNA that encodes mutant inverse pericam. However, the desired DNA plasmid is likely present at a low concentration, and moreover it is in nicked rather than intact circular form. What we would like to do now is repair and further amplify only the mutagenized product. Thankfully, we have E. coli bacteria to do this for us quite efficiently!
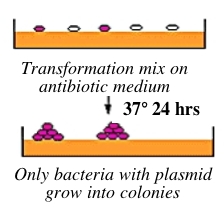
Bacteria can take up foreign DNA in a process called transformation, during which a single plasmid enters a bacterium and, once inside, replicates and expresses the genes it encodes. Most bacteria do not exist in a transformation-ready state, but can be made permeable to foreign DNA by chemical treatment or other means. Cells that are capable of transformation are referred to as competent; competent cells are extremely fragile and should be handled gently, i.e., kept cold and not vortexed. Bacterial transformation is efficient enough for most lab purposes, resulting in as many as 109 transformed cells per microgram of DNA, but even with highly competent cells only 1 DNA molecule in about 10,000 is successfully transformed. Thus we need a way to identify transformed cells, which is usually accomplished with antiobiotics. For example, the plasmid carrying inverse pericam (pRSET) also carries a gene that leads to ampicillin-resistance. Consequently, a transformed bacterium will grow on ampicillin-containing agar medium, while untransformed cells will die before they can form a colony. Given the low concentration and nicked structure of your DNA to begin with, you should perform your transformations today with great care.
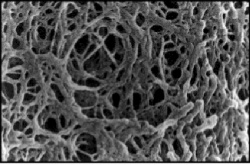
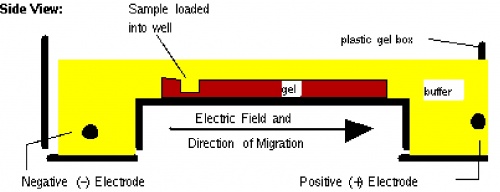
Before setting up transformations, you will test your mutagenized DNA for the presence and approximate concentration of product using gel electrophoresis. This technique separates large molecules by size using an applied electrical field and appropriate sieving matrix. DNA fragments are typically separated in gels composed of agarose, a seaweed-derived polymer (see figure, left). To prepare these gels, molten agarose is poured into a horizontal casting tray containing a comb. Once the agarose has solidified, the comb is removed, leaving wells into which the DNA sample can be loaded. The loaded DNA samples are then pulled through the matrix when a current is applied across it. Specifically, DNA molecules are negatively charged due to their phosphate backbones, and thus travel toward the positive charge at the far end of the gel (see figure, right).
Although all DNA molecules travel in the same direction during gel electrophoresis, they do so at different rates: larger molecules get entwined in the matrix and retarded, while smaller molecules wind through the matrix more easily and travel further from the well. Ultimately, fragments of similar length accumulate into “bands” in the gel. Bands of DNA are usually visualized by adding the fluorescent dye ethidium bromide to agarose gels. This dye intercalates between the bases of DNA, allowing DNA fragments to be located in the gel under UV light and photographed. The intensity of the band reflects the concentration of molecules that size, although there are upper and lower limits to the sensitivity of dyes. Because of its interaction with DNA, ethidium bromide is a powerful mutagen and will interact with the DNA in your body just as it does with any DNA on a gel. You should always handle all gels and gel equipment with nitrile gloves. Agarose gels with ethidium bromide must be disposed of as hazardous waste.
Today you will run your DpnI-digested mutagenesis reaction mixtures through an agarose gel. In each reaction, the long mutant plasmid DNA should be separated from the short digested fragments of parental DNA and thus can be identified. If you do not see a band at the expected size of the mutant plasmid, you might increase the amount of DNA used during the transformation procedure at the end of lab. In between electrophoresis and transformation, we will (most likely have visit from WAC today)…
Protocols
Part 1: Agarose gel electrophoresis
Using a 1% agarose gel prepared by the teaching faculty, you will run four samples as well as a reference lane of molecular weight markers (also called a DNA ladder).
- Set aside 10 μL of each of your DpnI-digested products – Y64D and X#Y – in eppendorf tubes. Save the rest of the digested DNA, keeping it on ice.
- Retrieve the two aliquots of undigested sample that you prepared last time, and add 2 μL of loading dye to all four samples.
- Loading dye contains xylene cyanol as a tracking dye to follow the progress of the electrophoresis (so you don’t run the smallest fragments off the end of your gel!) as well as glycerol to help the samples sink into the well.
- Flick the eppendorf tubes to mix the contents, then quick spin them in the microfuge to bring the contents of the tubes to the bottom.
- Load the gel in the order shown in the table below.
- To load your samples, draw 10 μL into the tip of your P20. Lower the tip below the surface of the buffer and directly over the well. You risk puncturing the bottom of the well if you lower the tip too far into the well itself (puncturing well = bad!). Expel your sample into the well. Do not release the pipet plunger until after you have removed the tip from the gel box (or you'll draw your sample back into the tip!).
- Once all the samples have been loaded, the teaching faculty will attach the gel box to the power supply and run the gel at 125 V for 45 minutes.
- While the gels run, you will here from the Writing Across the Curriculum teaching faculty. If you are done early, you can skip ahead to Part 4 of the protocol.
Part 2: Gel analysis=
- You will be shown how to photograph and save a digital image of your gel.
- In the following analysis, you will need the information for the 1 Kbp ladder you used, which is available at this link.
- First, see if you got a band at the expected size of inverse pericam in pRSET, or ~ 4 Kb
- If you did not get a band, consider preparing an extra transformation for that sample with 2-5x the usual recommended DNA amount.
- If you did get a band, estimate the approximate amount of DNA in that lane, then the concentration (based on the sample volume that you loaded). Keep this information in your notebook.
Part 3: Bacterial transformation
Each mutagenesis reaction (Y64D, and your chosen X#Y) will be transformed into competent cells called XL1-Blue and plated on separate Petri dishes. Tomorrow, two candidate colonies will be chosen from each plate. The efficiency of this mutagenesis protocol is reported to be 80 %. We will test two candidates per mutation to cover our bases, so to speak.
- Get an aliquot of competent cells from one of the teaching faculty. Keep these cells on ice at all times, allowing them to thaw slowly (over a few minutes).
- Label three 14 mL polypropylene round-bottom tubes as follows: (-) control Y64D, X#Y.
- Add 50 μL of competent cells to each tube, followed by 1 & m u ; L o f the appropriate D N A. Gently swirl (do not vortex) to mix, then incubate on ice for 10 minutes.
- Bring the tubes over to the 42 °C water bath, and immerse them for exactly 45 seconds according to your digital timer.
- Immediately return the cells to ice for 2 minutes, and take an aliquot of pre-warmed LB medium.
- Add 0.5 mL of warm LB to each sample, then move them to the 37 °C incubator. Ask the teaching faculty to show you how to operate the roller and balance your tubes.
- Allow the cells to recover and begin expressing ampicillin for 30 minutes. At the same time, pre-warm and dry three (or more, if needed) LB+AMP plates by placing them in the 37°C incubator, media side up with the lids ajar.
- Plate 250 μl of each transformation mix on LB+AMP plates. After dipping the glass spreader in the ethanol jar, you should pass it through the flame of the alcohol burner just long enough to ignite the ethanol. After letting the ethanol burn off, the spreader may still be very hot, and it is advisable to tap it gently on a portion of the agar plate without cells in order to equilibrate it with the agar (if it sizzles, it's way too hot). Once the plates are done, wrap them with colored tape and incubate them in the 37°C incubator overnight. One of the teaching faculty will remove them from the incubator and set up liquid cultures for you to use next time.
Part 4: Prepare tubes for liquid O/N cultures
You will make your teaching faculty very happy if you contribute to their preparatory work. Please label 4 large glass test tubes with your team color and sample name. Mix 12 ml LB with 12 μL of ampicillin. Aliquot 2.5 mL of LB+Amp per tube. These will be used to set up overnight cultures for you for next time.