Difference between revisions of "20.109(F21):M2D6"
Noreen Lyell (Talk | contribs) (→Introduction) |
Noreen Lyell (Talk | contribs) (→Protocols) |
||
| Line 16: | Line 16: | ||
| − | ===Part 3: | + | ===Part 3: Review journal article=== |
| − | + | Read and discuss the following journal article with your laboratory partner: | |
| − | + | Amberg-Johnson ''et al.'' "[[Media:Fa20 M2D5 paper discussion.pdf |Small molecule inhibition of apicomplexan FtsH1 disrupts plastid biogenesis in human pathogens.]]" ''eLife''. (2017) 6:e29865. | |
| − | + | ||
| − | + | The initial experiment presented by Amberg-Johnson ''et. al.'' shows the effect of actinonin on apicoplast biogenesis. The apicoplast is an essential plastid organ that is a key target for drug development in research focused on malaria treatment. Actinonin was identified in large-scale screen of compounds known to inhibit growth of parasite. The subsequent experiments completed in this research served to uncover the mechanism-of-action of actinonin is it pertains to disruption of the apicoplast. | |
| − | + | ||
| − | + | In the context of your research, this article focuses on the next step experiments that can be performed after a drug candidate is discovered from a screen. Though you can use this article as guidance as you consider the experiments that could follow your screen, remember that the specific next step experiments should be related to the protein target and drug candidate(s) identified in your project. For this exercise, the focus in on how the data are organized and presented. | |
| − | + | ||
| − | + | <font color = #4a9152 >'''In your laboratory notebook,'''</font color> complete the following with your partner: | |
| − | + | *Why is the apicoplast a promising target for anti-malarial drug development? | |
| − | + | *Why have attempts at developing broadly effective drugs that target the apicoplast been unsuccessful? | |
| − | + | *Why is the approach used by the researchers in this article more promising? | |
| − | + | *List the figures that are included in the article. For each figure: | |
| − | + | **What is the main conclusion / finding in each figure? | |
| − | + | **Which panel best supports the main conclusion / finding? Is more than one panel needed to fully support the main conclusion? | |
| − | + | **Are you convinced by the data? Do you agree with the main conclusion? | |
| − | + | *Are the figures organized in a coherent story? | |
| − | + | **Write transition statement that connect each figure to the next. A transition statement should very briefly summarize the findings of a figure and state what those findings motivated the research to do next (ie what is the next experiment?). | |
| − | + | ||
| − | # | + | |
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
==Reagents== | ==Reagents== | ||
Revision as of 04:17, 30 October 2021
Contents
Introduction
Interactions between low molecular weight ligands and proteins have been shown to increase the thermostability of proteins. This means that proteins bound to ligand are able to maintain tertiary structure, or resist denaturation, at higher temperatures than unbound proteins. Today we will use differential scanning fluorimetry (DSF) to examine the potential FKBP12 binders identified in our SMM screen.
DSF is a method used to identify low molecular weight ligands that bind and stabilize a protein of interest. In this assay, protein denaturation is measured via a fluorescent dye that has an affinity for hydrophobic regions. When the protein is folded the hydrophobic pockets are inaccessible to the dye and the fluorescent signal is quenched by water in the solution. As the protein unfolds, the dye interacts with the hydrophobic regions and emits a fluorescent signal that can be detected.
When a protein is bound to a ligand, the stability can be increased such that the temperature at which the protein denatures is increased. In the DSF assay, this is measured as a shift in the Tm, or melting temperature; which is defined as the temperature at which 50% of the protein is unfolded. This value represents the midpoint of the transition from structured (folded) to denatured (unfolded).
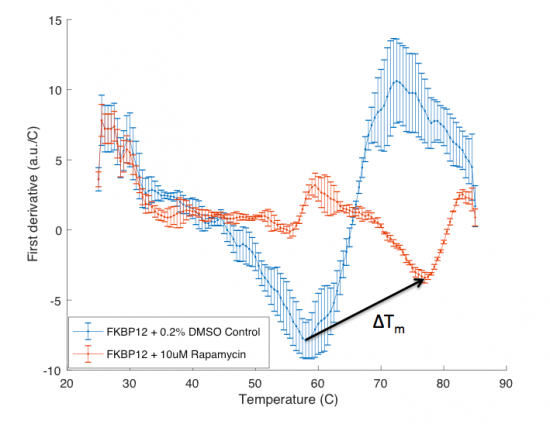
The ΔTm is the difference between the Tm of the unbound protein sample, or protein sample without added ligand, and the bound protein sample, protein sample with added ligand. If the tested ligand binds the protein of interest, the ΔTm can be observed as a shift in the plotted DSF data. For example, the data below show results of a pilot experiment completed in preparation for this module. In this graph the Tm of FKBP12 (blue curve) is ~50 °C. With the addition of rapamycin (red curve) the Tm is shifted to ~78 °C resulting in a ΔTm of ~20 degrees. Data in this plot was obtained by Becky Leifer from the Koehler lab.
Protocols
Part 3: Review journal article
Read and discuss the following journal article with your laboratory partner:
Amberg-Johnson et al. "Small molecule inhibition of apicomplexan FtsH1 disrupts plastid biogenesis in human pathogens." eLife. (2017) 6:e29865.
The initial experiment presented by Amberg-Johnson et. al. shows the effect of actinonin on apicoplast biogenesis. The apicoplast is an essential plastid organ that is a key target for drug development in research focused on malaria treatment. Actinonin was identified in large-scale screen of compounds known to inhibit growth of parasite. The subsequent experiments completed in this research served to uncover the mechanism-of-action of actinonin is it pertains to disruption of the apicoplast.
In the context of your research, this article focuses on the next step experiments that can be performed after a drug candidate is discovered from a screen. Though you can use this article as guidance as you consider the experiments that could follow your screen, remember that the specific next step experiments should be related to the protein target and drug candidate(s) identified in your project. For this exercise, the focus in on how the data are organized and presented.
In your laboratory notebook, complete the following with your partner:
- Why is the apicoplast a promising target for anti-malarial drug development?
- Why have attempts at developing broadly effective drugs that target the apicoplast been unsuccessful?
- Why is the approach used by the researchers in this article more promising?
- List the figures that are included in the article. For each figure:
- What is the main conclusion / finding in each figure?
- Which panel best supports the main conclusion / finding? Is more than one panel needed to fully support the main conclusion?
- Are you convinced by the data? Do you agree with the main conclusion?
- Are the figures organized in a coherent story?
- Write transition statement that connect each figure to the next. A transition statement should very briefly summarize the findings of a figure and state what those findings motivated the research to do next (ie what is the next experiment?).
Reagents
Introduction
Protocols
Reagent list
Next day: Complete data analysis