Difference between revisions of "20.109(F09): Mod 3 Day 5 Battery testing"
From Course Wiki
(→Introduction) |
(→Introduction) |
||
| Line 3: | Line 3: | ||
=<center>Battery Testing</center>= | =<center>Battery Testing</center>= | ||
==Introduction== | ==Introduction== | ||
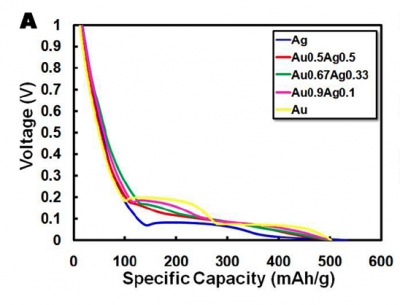
| − | [[Image:Charge-discharge.png|thumb|left|400px]]In order to determine the capacity of each battery we will be testing each battery on a Galvanostat. This is a machine that can apply either positive or negative current in order to either charge or discharge a battery with a specific amount of current. As an example, look at your cell phone battery, if you have a typical LiCoO2 | + | [[Image:Charge-discharge.png|thumb|left|400px]]In order to determine the capacity of each battery we will be testing each battery on a Galvanostat. This is a machine that can apply either positive or negative current in order to either charge or discharge a battery with a specific amount of current. As an example, look at your cell phone battery, if you have a typical LiCoO2 battery you will notice that it has a voltage (3.7 V) and a capacity (e.g. 710mAh for the Motorola MOTORAZR V3r battery). We know that LiCoO2 has a theoretical capacity of 130 mAh/g, so it's possible to back calculate the amount of active material to be 5.46 g of active material in my cell phone battery. The way Motorola determined the capacity and voltage for their cell phone battery is identical to what you will be doing in this lab, namely by attaching the battery to a Galvanostat and applying a constant current (1100 mA) in order to charge the battery in one hour, and then applying a negative current of 1100 mA to discharge the battery in one hour. The discharge graph shown here is an example of the curve that you will be generating. |
Once we know the capacity of the battery material, it's possible to determine the charge rate. We will be testing everything at 1C; this means that when we charge the battery it should take one hour, and it should discharge in one hour. Since every electrode weighs a little different, the charge/discharge rate will be slightly different. As an example, if I have an electrode of FePO4•H2O that has 1 mg of active material, then in order to discharge it in one hour I have to put a negative current of -143 uA. | Once we know the capacity of the battery material, it's possible to determine the charge rate. We will be testing everything at 1C; this means that when we charge the battery it should take one hour, and it should discharge in one hour. Since every electrode weighs a little different, the charge/discharge rate will be slightly different. As an example, if I have an electrode of FePO4•H2O that has 1 mg of active material, then in order to discharge it in one hour I have to put a negative current of -143 uA. | ||
<br><center>1 mg x143 mAh/g x 1 g/1000 mg x 1/1hr = 143uA</center><br> | <br><center>1 mg x143 mAh/g x 1 g/1000 mg x 1/1hr = 143uA</center><br> | ||
The electrodes that were prepared during last session were placed in a vacuum oven and dried at 100°C for four hours. They were then moved into a glove box and crimped into a battery. Today instruction will be given on how to run the battery on the Galvanostat. | The electrodes that were prepared during last session were placed in a vacuum oven and dried at 100°C for four hours. They were then moved into a glove box and crimped into a battery. Today instruction will be given on how to run the battery on the Galvanostat. | ||
Revision as of 17:50, 27 November 2009
Battery Testing
Introduction
In order to determine the capacity of each battery we will be testing each battery on a Galvanostat. This is a machine that can apply either positive or negative current in order to either charge or discharge a battery with a specific amount of current. As an example, look at your cell phone battery, if you have a typical LiCoO2 battery you will notice that it has a voltage (3.7 V) and a capacity (e.g. 710mAh for the Motorola MOTORAZR V3r battery). We know that LiCoO2 has a theoretical capacity of 130 mAh/g, so it's possible to back calculate the amount of active material to be 5.46 g of active material in my cell phone battery. The way Motorola determined the capacity and voltage for their cell phone battery is identical to what you will be doing in this lab, namely by attaching the battery to a Galvanostat and applying a constant current (1100 mA) in order to charge the battery in one hour, and then applying a negative current of 1100 mA to discharge the battery in one hour. The discharge graph shown here is an example of the curve that you will be generating.Once we know the capacity of the battery material, it's possible to determine the charge rate. We will be testing everything at 1C; this means that when we charge the battery it should take one hour, and it should discharge in one hour. Since every electrode weighs a little different, the charge/discharge rate will be slightly different. As an example, if I have an electrode of FePO4•H2O that has 1 mg of active material, then in order to discharge it in one hour I have to put a negative current of -143 uA.
The electrodes that were prepared during last session were placed in a vacuum oven and dried at 100°C for four hours. They were then moved into a glove box and crimped into a battery. Today instruction will be given on how to run the battery on the Galvanostat.