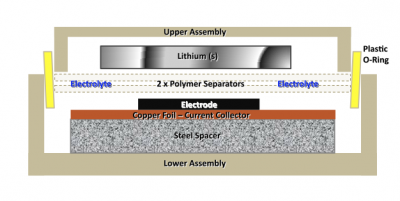
20.109(F09): Mod 3 Day 4 Battery assembly
From Course Wiki
Battery Assembly
Introduction
Protocols
All material should be isolated and dried to form a powder. This powder is what will be used to make the battery electrodes. In all battery electrodes there is a certain percentage of active material, a small amount of conducting material and a small amount of binding material. In these batteries, the conducting material will be a particular type of graphite carbon called “Super P”. The binder is a compound that causes all the powder to stick together and form a flexible film, the binder is teflon (poly(tetrafluoroethylene)—PTFE). These will be mixed at a ratio of 70% active material, 25% Super P, and 5% PTFE.
- Measure the mass of powder and add to a clean agate mortar, determine the mass of Super P and PTFE to add based on this mass.
- Measure out Super P and add to mortar. Do not add PTFE yet!
- Grind thoroughly with mortar and pestle for about 15 minutes.
- Add PTFE and mix together, use the pestle to press the mixture into a flat sheet that is all one piece.
- Transfer to a stainless steel plate and roll out the electrode to form a uniform electrode.
- Once rolled, transfer to the stainless steel cutting plate and use a circle cutter to make an electrode.
- Measure mass of electrode and determine the theoretical capacity of the electrode. Co3O4 has a theoretical capacity of ~890mAh/g, FePO4•H2O has a capacity of 143 mAh/g (milli Amp hours per gram).